Xiaosheng Hang1, Qing Sun2, Weidong Mao3, Baoliang Zhong4, Haiyuan Xu5,
Wenxiang Shen5, Steven Jay Tucker6, Daoyuan Wang7
1Department of Oncology, Wuxi Cancer Hospital, Medical School of Suzhou University, Jiangsu Province, PR China.
2Department of Oncology, Wuxi 2nd Hospital, Nanjing Medical University, Jiangsu Province, PR China.
3Department of Oncology, Jiangyin People’s Hospital, Medical School of Dongnan University, Jiangsu Province, PR China.
4School of Public Health, Peking University, No. 38 Xueyuan Rd, Beijing 100083, PR China.
5Department of Oncology, The First Kunshan People’s Hospital, Medical School of Jiangsu University, Jiangsu Province, PR China.
6Pacific Cancer Centre, 290 Orchard Road #19-01, Singapore 238859.
7AmMed Cancer Center, Ruijin Hospital, Medical School of Shanghai Jiao tong University, Shanghai 200025, PR China.
ABSTRACT
OBJECTIVE To investigate the efficacy and safety of combination chemotherapy for patients with advanced gastric cancer.
METHODS Treatment consisted of paclitaxel (75 mg/m2) and leucovorin (40 mg/m2) as a 2-h intravenous infusion, followed by 5-fluorouracil (2,400 mg/m2) as a 46-h continuous infusion. Cycles were repeated every 2 weeks.
RESULTS Forty-six (93.9%) of the 49 patients were evaluable for response. One case of CR and 21 cases of PR were confirmed, giving an overall response rate of 44.9% (95% CI, 31.0-58.8%). The median TTP for all patients was 5.3 (range 1.5–9.0) months. The estimated median overall survival was 10.3 months (range 2.9–28.5 months). The most common toxicity was grade 1–2 anemia (80.4% of patients) and anorexia (65.2% of patients).
CONCLUSION Combination of biweekly paclitaxel and infusional 5-fluorouracil and leucovorin is an effective regimen with excellent tolerability as first-line treatment of advanced gastric cancer.
KEYWORDS Gastric cancer; Paclitaxel; 5-Fluorouracil; Leucovorin; Biweekly
INTRODUCTION
Although the incidence of gastric cancer has decreased in most western countries, it remains a significant problem in global health terms and is the second most common cause of cancer mortality worldwide, and a leading cause of cancer death in Asian countries. Surgery is the only potentially curative treatment for localized gastric cancer, but most cases present at an advanced stage. The prognosis for the disease is extremely poor, with overall 5-year survival rates ranging from 10% to15% in the United States and most developed countries [1].
The efficacy of chemotherapy with palliative intent compared with supportive care alone is now widely accepted [2]. Studies showed the benefit of combination regimens, such as fluorouracil (FU), doxorubicin, and methotrexate (FAMTX) [3,4] or etoposide, leucovorin (LV), and FU(ELF) [5], over best supportive care [6]. The survival advantage is small, however, and no internationally accepted standard regimen has emerged [7].
Paclitaxel, derived from the bark of the Pacific yew, Taxus brevifolia, is one of the most active anticancer drugs for the treatment of solid tumors, effectively blocking cancer cells in the G2/M phase through the inhibition of microtubular depolymerization [8]. Since paclitaxel is known to be a cell-cycle-specific agent, in vitro experiments have suggested that prolonged exposure to paclitaxel, through either continuous infusion schedules or weekly administration, can lead to enhanced cytotoxicity [9]. Furthermore, recent clinical studies have demonstrated that weekly schedules of intravenous paclitaxel have promising antitumor activity with tolerable safety profiles for several types of solid tumors, including lung, breast, and ovarian cancer [10-12]. When administered alone, paclitaxel showed a 20–23% objective response rate in patients with advanced gastric cancer [13,14]. In addition, paclitaxel acts synergistically with 5-fluorouracil in a sequence dependent manner. Thus, paclitaxel followed by 5-fluorouracil was highly synergistic, while pre-exposure to 5-fluorouracil followed by paclitaxel resulted in marked antagonism [15,16]. The non-overlapping toxicity profile of paclitaxel and infusional 5-fluorouracil, and the schedule-dependent synergism between these drugs against human gastric cancer cells [15,16] suggest the potential value of investigation of such a combination in the treatment of patients with advanced gastric cancer. For example, Murad et al. reported a response rate of 66% in a phase II trial treated with 5-FU continuous infusion and paclitaxel every 3 weeks [17]. Bokemeyer et al. treated chemotherapy-naïve gastric cancer with weekly 5-FU plus leucovorin combined with paclitaxel and obtained a response rate of 32% and overall survival of 11 months [18]. Based on these results, we conducted a multi-center phase II trial of combination chemotherapy with biweekly paclitaxel plus infusional 5-FU and leucovorin to evaluate the response rate, time to progression, and safety of this regimen in advanced gastric cancer.
PATIENTS AND METHODS
Eligibility
All the patients involved in the current study had histologically confirmed metastatic or recurrent gastric adenocarcinoma with at least one unidimensional measurable lesion (i.e., a diameter ≥1 cm, as assessed by spiral computed tomography). The patients were 18–75 years of age with a performance status of 0–2 on the Eastern Cooperative Oncology Group (ECOG) scale. Plus, adequate hematological (absolute neutrophil count ≥1.5×109/l, platelet count ≥100×109/l, hemoglobin ≥9 g/dl), renal (serum creatinine ≤1.5mg/dl and creatinine clearance ≥50 ml/min), and hepatic (total bilirubin ≤2.0 mg/dl and serum transaminase level ≤3 times the upper limit of the normal range) levels were also required. Patients who had received adjuvant chemotherapy completed 4 weeks before entry were eligible. Patients were ineligible if they had previously received palliative chemotherapy or radiation therapy, or had other severe medical illnesses, CNS metastasis, another active malignancy, or history of anaphylaxis to drugs. The institutional review board of each author’s institution approved the protocol, and written informed consent was obtained from all patients before enrollment.
Study treatment
Chemotherapy consisted of paclitaxel (Fuwang; Yangtze River Pharm. Co., Ltd. Jiangsu Province, China) at 75 mg/m2 and leucovorin at 40 mg/m2 as a 2-h intravenous infusion, followed by 5-fluorouracil at 2,400 mg/m2 as a 46-h continuous infusion. Cycles were repeated every 2 weeks. Treatment was continued for up to 24 weeks unless the disease progressed, unacceptable toxicity developed, or the patient refused continued treatment. Treatment delay and dose modification were based on the worst adverse effects observed during the previous cycle.
Study assessments
A screening assessment, including a medical history, physical examination, ECG, chest X-ray, and tumor assessment, was conducted within 2 weeks before starting treatment. Further assessments conducted within 7 days before starting treatment included vital signs, ECOG performance status, and laboratory tests. Complete blood counts were performed weekly during the first cycle and every cycle thereafter, and biochemical tests performed before each cycle. Tumors were measured every two cycles until the tumor progressed. The tumor responses were classified according to the response evaluation criteria in solid tumors (RECIST) guidelines. Patients with a complete response (CR) or partial response (PR) required a confirmatory disease assessment at least 4 weeks later. Adverse events were graded according to National Cancer Institute Common Toxicity Criteria (NCI-CTC) version 2.0.
Statistical analysis
The current trial used a two-stage optimal design, as proposed by Simon, with an 80% power to accept the hypothesis and 5% significance to reject the hypothesis [19]. Plus, the current trial was designed to detect a response rate of 40% as compared to a minimal, clinically meaningful response rate of 20%. Allowing for a follow-up loss rate of 10%, the total sample size was 48 patients with a measurable disease. All enrolled patients were included in the intention-to-treat analysis of efficacy. The duration of response, time to progression (TTP), and survival analyses were all estimated using the Kaplan–Meier method. The duration of response was defined as the interval from the onset of a CR or PR until evidence of disease progression was found. Meanwhile, the TTP was calculated from the initiation of chemotherapy to the date of disease progression, while overall survival was measured from the initiation of chemotherapy to the date of the last follow-up or death. The statistical data were obtained using a SPSS software package (SPSS 11.5 Inc. Chicago, IL, USA).
RESULTS
Patient characteristics
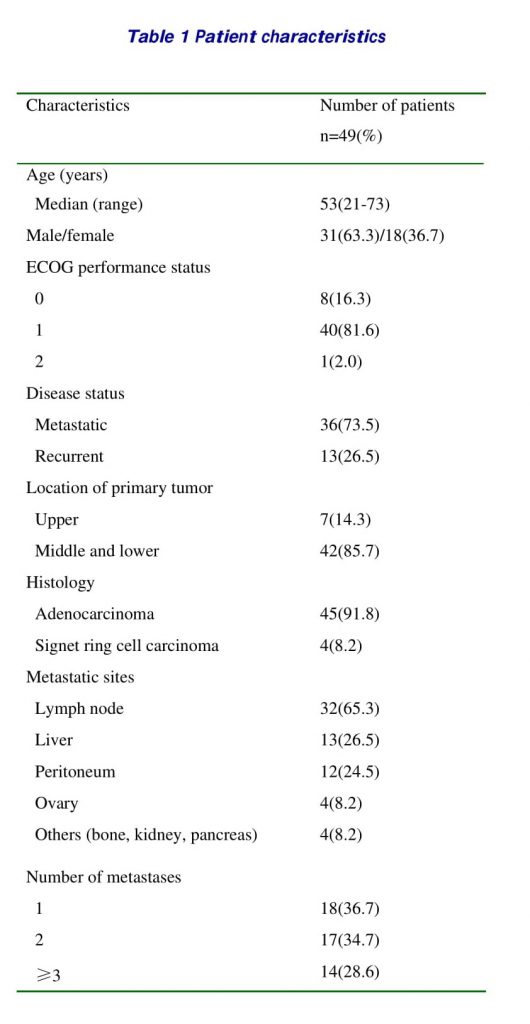
From July 2005 to December 2006, a total of 49 patients were enrolled in the current study from four centers. The characteristics of the patients are summarized in Table 1. The median age was 53 (range, 21–73) years, with 31 males and 18 females. Most of the patients (98.0%) had a good performance status (ECOG 0 or 1). Thirty-six (73.5%) patients had a metastatic disease, while 13 patients had a recurrent disease after surgical resection (total or subtotal gastrectomy) of the primary tumor. Distal lymph nodes and the liver were the most common sites of the metastases. No patients had received prior chemotherapy or radiotherapy.
Efficacy and Survival
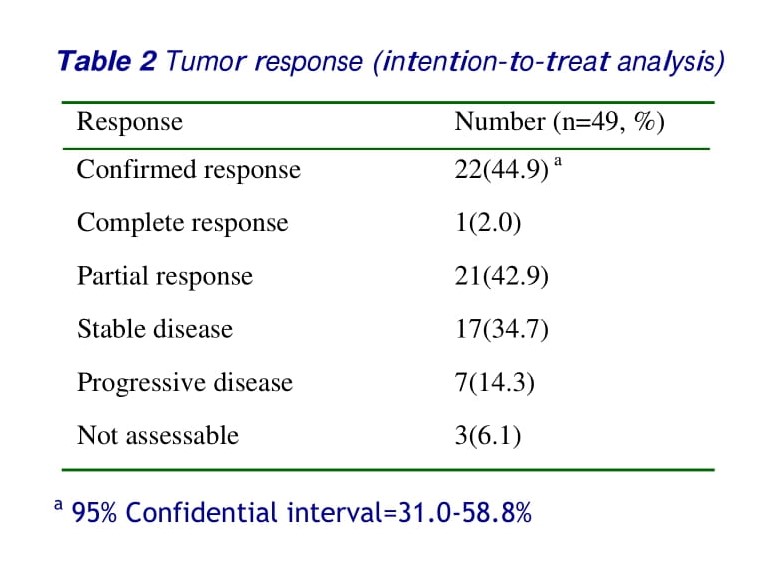
Forty-six (93.9%) of the 49 patients were assessable for response, of the three patients not assessable, two were lost to follow-up after the first cycle of the treatment, and the other died after the first cycle of unknown cause, although brain metastasis was suspected. All efficacy data are reported using the intent- to-treat patient population. One case of CR and 21 cases of PR were confirmed, giving an overall response rate of 44.9% (95% CI, 31.0-58.8%). See table 2.
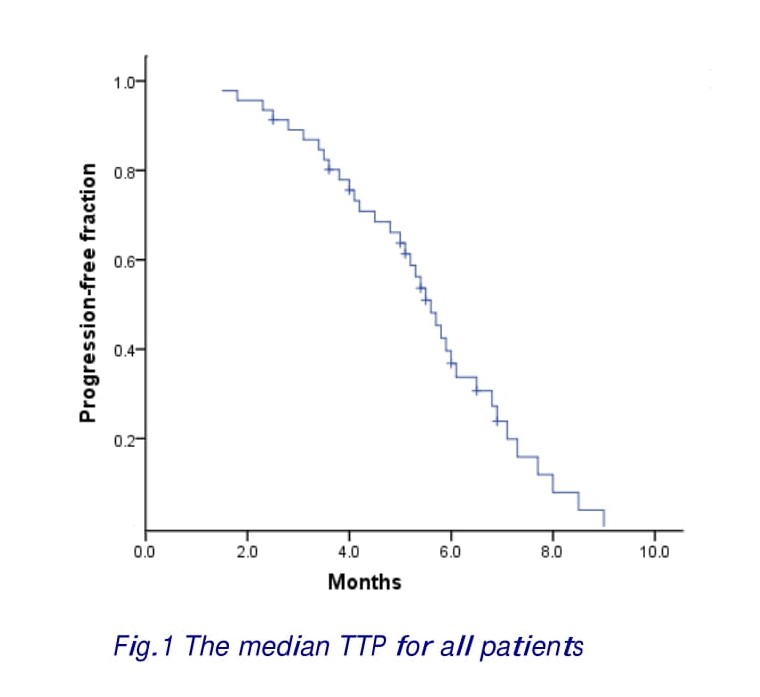
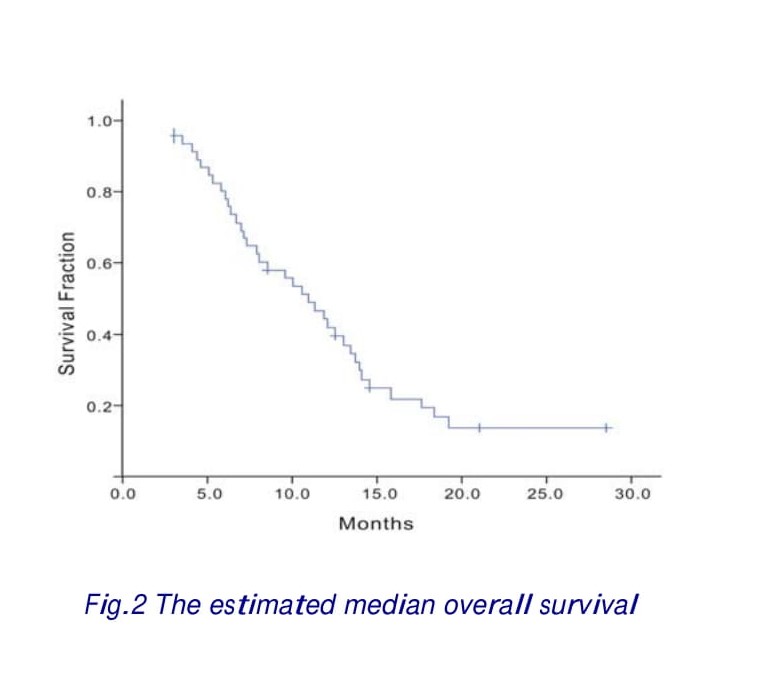
Of 22 responses, 15 (68.2%) were observed after four cycles, 4 (18.2%) after six cycles and 3 (13.6%) after eight cycles of chemotherapy. The median follow-up period was 11.6 months. The median TTP for all patients was 5.3 (range 1.5–9.0) months (Fig. 1). Twenty-one patients (45.6%) received a second-line therapy, such as irinotecan, capecitabine, or oxaliplatin after disease progression. Twenty-four patients had died at the time of the present evaluation. The estimated median overall survival was 10.3 months (range 2.9-28.5 months) (Fig.2).
Toxicity
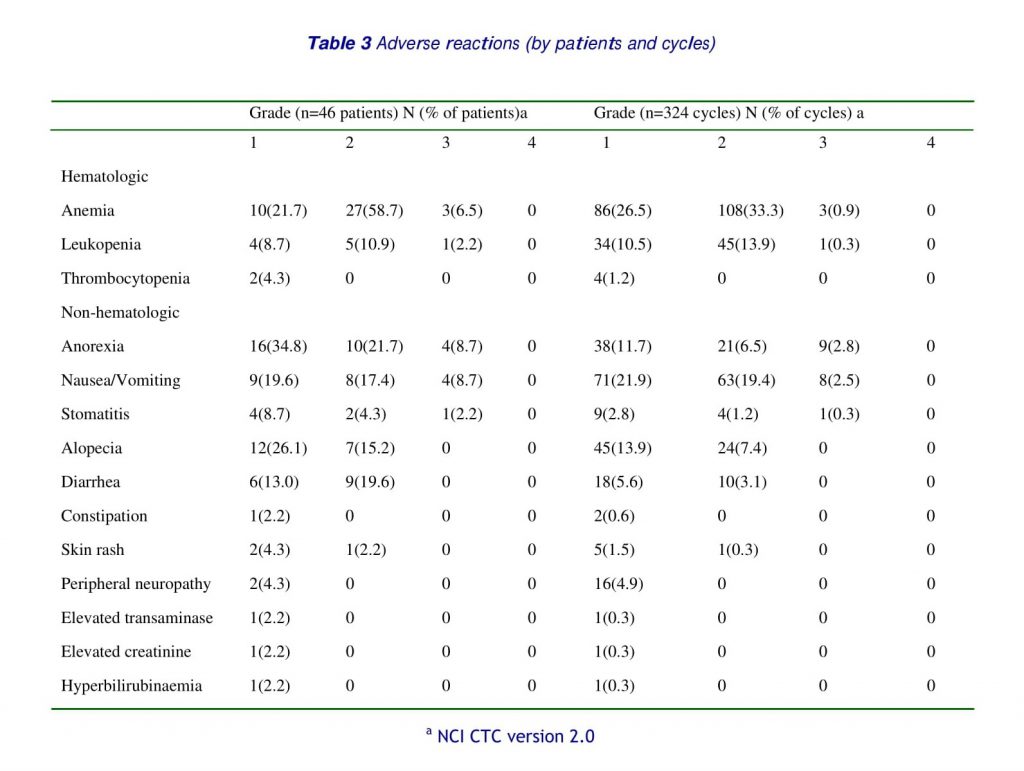
A total of 324 cycles were administered, with a median of 7 cycles per patient (range 1–10 cycles). The occurrence and the incidence of toxicities are shown in Table 3. No cases of grade 4 toxicity were observed. The most common toxicities were gastrointestinal. Grade 1 and 2 anorexia, nausea/vomiting, diarrhea, and stomatitis were reported in 26(56.5%), 17(37.0%), 15(32.6%) and 6(13.0%) cases in the study population, and in 59(18.2%), 134(41.4%), 28(8.6%) and 13(4.0%) cycles, respectively. Grade 3 anorexia, nausea/vomiting, and stomatitis were recorded in only 4(8.7%), 4(8.7%), and 1(2.2%) case, and in 9(2.8%), 8(2.5%), and 1(0.3%) cycle, respectively. Severe hematological toxicity was uncommon. Grade 3 anemia, leukopenia, and thrombocytopenia were noted in 3(6.5%), 1(2.2%) and 0 cases, and 3(0.9%), 1(0.3%) and 0 cycles, respectively. Mild to moderate alopecia was observed in 19(41.3%) cases, grade 1 in 12(26.1%) and grade 2 in 7(15.2%) cases, and 69(21.3%) cycles, grade 1 in 45(13.9) and grade 2 in 24 (7.4%) cycles, respectively. There were no febrile neutropenia or treatment-related deaths in this series.
DISCUSSION
In advanced gastric cancer, systemic chemotherapy has been tried as a palliative purpose, leading to improvement of tumor responses, quality of life and survival compared to best supportive care [7]. However, the standard chemotherapy has not yet been established. We considered paclitaxel as a mainstay agent and combined 5-FU and leucovorin onto it. The rationale behind this strategy was as follows: (1) the synergistic effect of the combination; (2) no overlapping toxicity; (3) recent results of 5-FU and docetaxel combination and (4) less myelotoxicity of paclitaxel than docetaxel [20]. Continuous infusion of 5-FU combined with leucovorin has been an acceptable regimen in gastrointestinal malignancy [21]. It was evaluated as a salvage therapy for patients having a poor general condition such as acute disseminated intravascular coagulation (DIC) [15].
The results of this study indicated that biweekly paclitaxel plus infusional 5-fluorouracil and leucovorin is an active combination chemotherapy regimen for patients with advanced gastric cancer, providing excellent tolerability. The overall response rate of 44.9% (95% CI, 31.0-58.8%) and median overall survival of 8.8 months (range 2.7–29.3 months) were within the ranges achieved by previously reported major protocols, such as FAM (5-FU, doxorubicin and mitomycin) [22], EAP (etoposide, doxorubicin and cisplatin) [23], ELF (etoposide, LV and 5-FU) [24], FAMTX (5-FU, doxorubicin and high-dose methotrexate) [4], FP (5-FU and cisplatin) [25], ECF (epirubicin, cisplatin and 5-FU continuous infusion) [26], FLP (5-FU, LV and cisplatin) [27], PELF (cisplatin, epirubicin, LV and 5-FU) [28], PE-HDFL (cisplatin, etoposide, and weekly high-dose 5-FU and LV infusion) [29], and recent oxaliplatin-based [30-32] or irinotecan-based regimens [33-35].
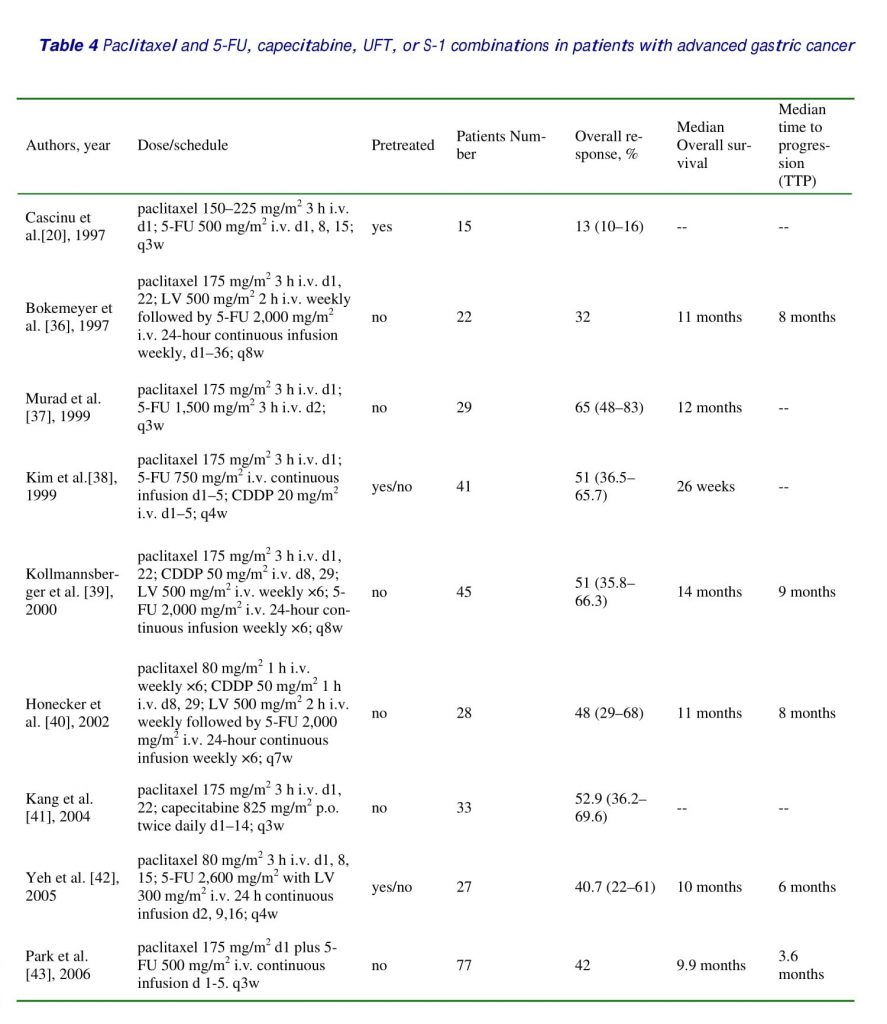
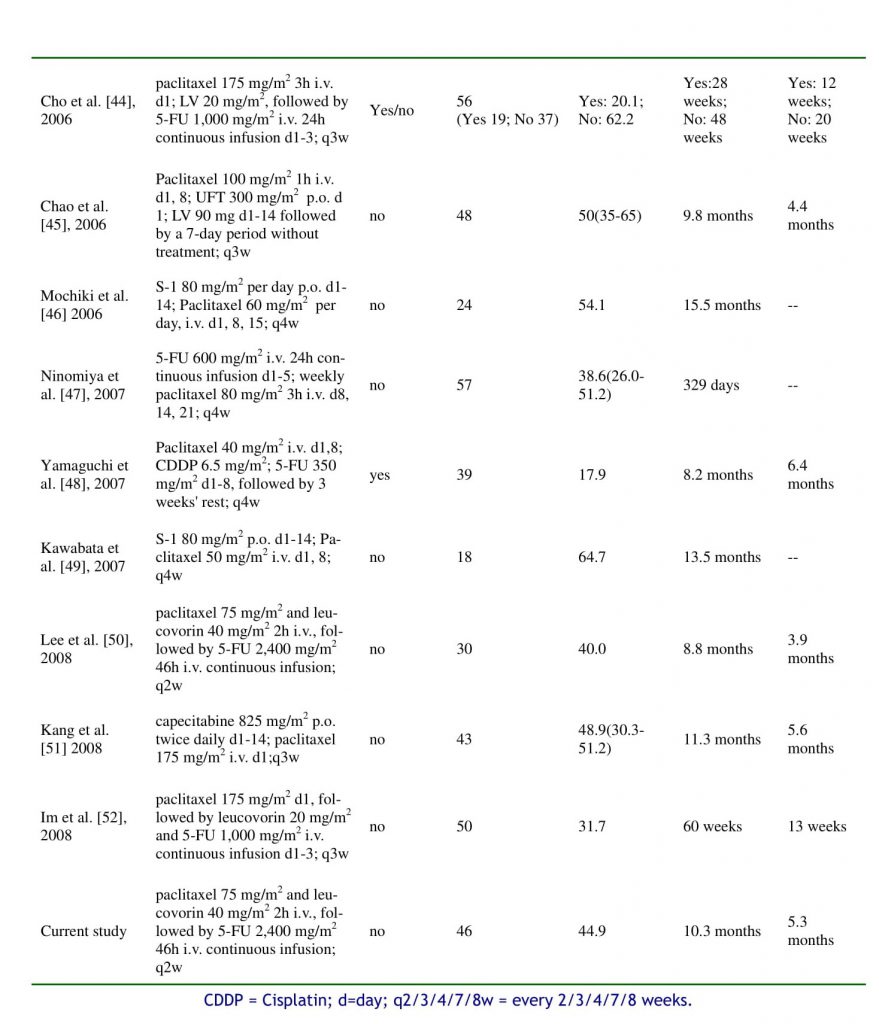
Recently, other series of studies using different administration schedules of paclitaxel and 5-FU have also demonstrated good efficacy in gastric cancer [36–53]. Paclitaxel appears to be a useful agent in systemic chemotherapy against advanced gastric cancer. Generally, other series of studies using different administration schedules of paclitaxel and 5-FU or 5-FU pro-drugs (capecitabine, UFT or S-1), have demonstrated comparable efficacy with that of the current study [36–52] (listed in table 4). Some of them studied chemo-naive patients and used only first-line treatment [36,37,39-41,43,45-47,49-52], some of them added cisplatin into paclitaxel/5-FU combinations [38-40, 48], some of them used 3-weekly paclitaxel [20, 36-39,41,43,44,51,52], some of them used weekly paclitaxel [40,42,45-49], some of them used biweekly paclitaxel[50], and some reported the experience of a single patient [53]. It is impossible and irrelevant to make comparisons between these phases I, I/II, or II studies or a case report.
Paclitaxel shows good activity against advanced gastric cancer using different administration schedules and various combination regimens with other chemotherapeutic agents [54,55]. Single-agent paclitaxel regimens, using 3- and 24-h infusion, produced response rates of 8 and 20%, respectively, in the treatment of advanced gastric cancer [56]. In combination with 5-fluorouracil and/or platinum compounds, response rates ranging from 32 to 65.5% were reported in several trials conducted in different settings [23, 41, 43–45, 50,57, 58]; some delivered paclitaxel weekly, others every 3 weeks; some introduced cisplatin or carboplatin in combination with paclitaxel and 5-fluorouracil or used oral fluoropyrimidine; some studied chemonaive patients and/or previously treated patients. Relatively high incidences (14–45%) of grade 3 or 4 neutropenia were significant adverse effects in these studies [41, 44,57, 58], which can result in treatment-related morbidity or mortality, thereby compromising the patients’ quality of life and increasing medical costs.
In our multi-center prospective study, biweekly paclitaxel with infusional 5-fluorouracil and leucovorin showed a modest response rate and median overall survival time comparable to those achieved by previously reported paclitaxel-based weekly or 3-weekly regimens [41,44,57,58]. In addition, with respect to adverse reactions, this biweekly paclitaxel-based approach showed a more favorable toxicity profile as compared with weekly or 3-weekly paclitaxel-based regimens [41, 43, 44, 50, 57, 58]. There was no grade 4 toxicity or treatment-related death in this study. The most common adverse reaction was grade 3 gastrointestinal toxicity such as anorexia, nausea/vomiting, and stomatitis, which were reported in 4(8.7%), 4(8.7%), and 1(2.2%) case, and in 9(2.8%), 8(2.5%), and 1(0.3%) cycle, respectively. Grade 3 hematological toxicity was observed in 4(8.7%) cases, include 3(6.5%) anemia and 1(2.2%) leucopenia, and 3(0.9%), 1(0.3%) cycle, respectively, with no severe treatment-related morbidity such as febrile neutropenia.
CONCLUSION
We conclude that the combination of biweekly paclitaxel and infusional 5-fluorouracil and leucovorin is an active regimen with excellent tolerability as first-line treatment of advanced gastric cancer, especially in elderly patients or those in poor condition. Although modest effects were shown, further trials are needed to determine the optimal paclitaxel dose and combination schedule to improve treatment outcome.
REFERENCE
1. Karpeh MS, Kelsen DP, Tepper JE. Cancer of the stomach, in DeVita VT, Hellman S, Rosenberg SA (eds): Cancer: Principles and Practice on Oncology (ed 6). Philadelphia, PA, Lippincott, Williams & Wilkins, 2001, pp 1092-1126.
2. Kohne CH, Wils JA, Wilke HJ. Developments in the treatment of gastric cancer in Europe. Oncology (Williston Park). 2000; 14(12 Suppl 14):22-25.
3. Waters JS, Norman A, Cunningham D, Scarffe JH, Webb A, Harper P, et al. Long-term survival after epirubicin, cisplatin and fluorouracil for gastric cancer: Results of a randomized trial. Br J Cancer 1999; 80(1-2):269-272.
4. Wils JA, Klein HO, Wagener DJ, Bleiberg H, Reis H, Korsten F, et al. Sequential high-dose methotrexate, and fluorouracil combined with doxorubicin: A step ahead in the treatment of advanced gastric cancer—A trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cooperative Group. J Clin Oncol 1991; 9(5):827-831.
5. Vanhoefer U, Rougier P, Wilke H, Ducreux MP, Lacave AJ, Van Cutsem E, et al. Final results of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: A trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. J Clin Oncol 2000; 18(14): 2648-2657.
6. Glimelius B, Ekström K, Hoffman K, Graf W, Sjödén PO, Haglund U, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol 1997; 8(2):163-168.
7. Ajani JA. Standard chemotherapy for gastric carcinoma: Is it a myth? J Clin Oncol 2000; 18(23): 4001-4003.
8. Jordan MA, Wendell K, Gardiner S, Derry WB, Copp H, Wilson L. Mitotic block induced in HeLa cells by low concentrations of paclitaxel (Taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res 1996; 56(4):816–825.
9. Zhan Z, Scala S, Monks A, Hose C, Bates S, Fojo T. Resistance to paclitaxel mediated by P-glycoprotein can be modulated by changes in the schedule of administration. Cancer Chemother Pharmacol 1997; 40(3):245–250.
10. Akerley W, Herndon JE, Egorin MJ, Lyss AP, Kindler HL, Savarese DM, et al. Weekly, high-dose paclitaxel in advanced lung carcinoma: a phase II study with pharmacokinetics by the Cancer and Leukemia Group B. Cancer 2003; 97(10): 2480–2486.
11. Seidman AD, Hudis CA, Albanell J, Tong W, Tepler I, Currie V, et al. Dose-dense therapy with weekly 1-hour paclitaxel infusions in the treatment of metastatic breast cancer. J Clin Oncol 1998; 16(10): 3353–3361.
12. Markman M, Hall J, Spitz D, Weiner S, Carson L, Van Le L, et al. Phase II trial of weekly single-agent paclitaxel in platinum/paclitaxel-refractory ovarian cancer. J Clin Oncol 2002; 20(9): 2365–2369.
13. Ohtsu A, Boku N, Tamura F, Muro K, Shimada Y, Saigenji K, et al. An early phase II study of a 3-hour infusion of paclitaxel for advanced gastric cancer. Am J Clin Oncol 1998;21(4): 416–419.
14. Yamada Y, Shirao K, Ohtsu A, Boku N, Hyodo I, Saitoh H, et al. Phase II trial of paclitaxel by three-hour infusion for advanced gastric cancer with short premedication for prophylaxis against paclitaxel-associated hypersensitivity reactions. Ann Oncol 2001; 12(8): 1133–1137
15. Cascinu S, Ficarelli R, SaW MA, Graziano F, Catalano G, Cellerino R. A phase I study of paclitaxel and 5-Xuorouracil in advanced gastric cancer. Eur J Cancer 1997; 33(10): 1699–1702.
16. Grem JL, Nguyen D, Monahan BP, Kao V, GeoVroy FJ. Sequence-dependent antagonism between Xuorouracil and paclitaxel in human breast cancer cells. Biochem Pharmacol 1999; 58(3): 477–486.
17. Murad AM, Petroianu A, Guimaraes RC, Aragao BC, Cabral LO, Scalabrini-Neto AO. Phase II trial of the combination of paclitaxel and 5-fluorouracil in the treatment of advanced gastric cancer: a novel, safe, and effective regimen. Am J Clin Oncol 1999; 22(6): 580-586
18. Bokemeyer C, Hartmann JT, Lampe CS, Clemens MR, Quietzsch D, Forkmann L, et al. Paclitaxel and weekly 24-h infusion of 5-fluorouracil/folinic acid in advanced gastric cancer. Semin Oncol 1997; 24(6 Suppl 19): S19-96-S19-100.
19. Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials 1989; 10(1):1–10.
20. Yeh KH, Cheng AL, Lin JF, Hsu CH, Lu YS, Chen YC. Sequence dependent synergism of paclitaxel and 5-Xuorouracil in the treatment of gastric cancer: In vitro and pilot clinical studies (abstract 225). Ann Oncol 1998; 9: S47.
21. Vanhoefer U, Wilke H, Weh HJ, Clemens M, Harstrick A, Stahl M, et al. Weekly high-dose 5-Xuorouracil and folinic acid as salvage treatment in advanced gastric cancer. Ann Oncol 1994; 5(9): 850 851.
22. MacDonald JS, Schein PS, Woolley PV, Smythe T, Ueno W, Hoth D, et al. 5-Fluorouracil, doxorubicin, and mitomycin (FAM) combination chemotherapy for advanced gastric cancer. Ann Intern Med 1980; 93(4):533–536.
23. Preusser P, Wilke H, Achterrath W, Fink U, Lenaz L, Heinicke A, et al. Phase II study with the combination etoposide, doxorubicin, and cisplatin in advanced measurable gastric cancer. J Clin Oncol 1989; 7(9):1310–1317.
24. Wilke H, Preusser P, Fink U, Achterrath W, Lenaz L, Stahl M, et al. High dose folinic acid/etoposide/5-fluorouracil in advanced gastric cancer – A phase II study in elderly patients or patients with cardiac risk. Invest New Drugs 1990; 8 (1): 65–70.
25. Kim NK, Park YS, Heo DS, Suh C, Kim SY, Park KC, et al. A phase III randomized study of 5-fluorouracil and cisplatin versus 5-fluorouracil, doxorubicin, and mitomycin C versus 5-fluorouracil alone in the treatment of advanced gastric cancer. Cancer 1993; 71(12): 3813–3818.
26.Zaniboni A, Barni S, Labianca R, Marini G, Pancera G, Giaccon G, et al. Epirubicin, cisplatin, and continuous infusion 5-fluorouracil is an active and safe regimen for patients with advanced gastric cancer. An Italian Group for the Study of Digestive Tract Cancer (GISCAD) report. Cancer 1995; 76 (10):1694–1699.
27. Ychou M, Astre C, Rouanet P, Fabre JM, Saint-Aubert B, Domergue J, et al. A phase II study of 5-fluorouracil, leucovorin and cisplatin (FLP) for metastatic gastric cancer. Eur J Cancer 1996; 32A (11):1933–1937.
28. Cocconi G, Bella M, Zironi S, Algeri R, Di Costanzo F, De Lisi V, et al. Fluorouracil, doxorubicin, and mitomycin combination versus PELF chemotherapy in advanced gastric cancer: A prospective randomized trial of the Italian Oncology Group for Clinical Research. J Clin Oncol 1994; 12(12): 2687–2693.
29. Cheng AL, Yeh KH, Lin JT, Hsu C, Liu MY. Cisplatin, etoposide, and weekly high-dose 5-fluorouracil and leucovorin infusion (PEHDFL)–A very effective regimen with good patients’ compliance for advanced gastric cancer. Anticancer Res 1998; 18(2B): 1267–1272.
30. Louvet C, Andre T, Tigaud JM, Gamelin E, Douillard JY, Brunet R, et al. Phase II study of oxaliplatin, fluorouracil, and folinic acid in locally advanced or metastatic gastric cancer patients. J Clin Oncol 2002; 20(23): 4543–4548.
31. Kim DY, Kim JH, Lee SH, Kim TY, Heo DS, Bang YJ, et al. Phase II study of oxaliplatin, 5-fluorouracil and leucovorin in previously platinum-treated patients with advanced gastric cancer. Ann Oncol 2003; 14(3): 383–387.
32. Chao Y, Yeh KH, Chang CJ, Chen LT, Chao TY, Wu MF, et al. Phase II study of weekly oxaliplatin and 24-h infusion of high dose 5-Xuorouracil and folinic acid in the treatment of advanced gastric cancer. Br J Cancer 2004; 91(3): 453–458.
33. Bleiberg H. CPT-11 in gastrointestinal cancer. Eur J Cancer 1999; 35(3): 371–379.
34. Ajani JA, Baker J, Pisters PW, Ho L, Mansfield PF, Feig BW, et al. CPT-11 plus cisplatin in patients with advanced, untreated gastric or gastroesophageal junction carcinoma: Results of a phase II study. Cancer 2002; 94(3): 641–646.
35. Slater S, Shamash J, Wilson P, Gallagher CJ, Slevin ML. Irinotecan, cisplatin and mitomycin in inoperable gastroesophageal and pancreatic cancers – A new active regimen. Br J Cancer 2002; 87(8): 850–853.
36. Bokemeyer C, Lampe CS, Clemens MR, Hartmann JT, Quietzsch D, Forkmann L, et al. A phase II trial of paclitaxel and weekly 24 h infusion of 5-fluorouracil/folinic acid in patients with advanced gastric cancer. Anticancer Drugs 1997; 8(4): 396–399.
37. Murad AM, Petroianu A, Guimaraes RC, Aragao BC, Cabral LO, Scalabrini-Neto AO. Phase II trial of the combination of paclitaxel and 5-fluorouracil in the treatment of advanced gastric cancer: A novel, safe, and effective regimen. Am J Clin Oncol 1999; 22(6): 580–586.
38. Kim YH, Shin SW, Kim BS, Kim JH, Kim JG, Mok YJ, et al. Paclitaxel, 5-fluorouracil, and cisplatin combination chemotherapy for the treatment of advanced gastric carcinoma. Cancer 1999; 85(2): 295–301.
39. Kollmannsberger C, Quietzsch D, Haag C, Lingenfelser T, Schroeder M, Hartmann JT, et al. A phase II study of paclitaxel, weekly, 24-hour continuous infusion 5-fluorouracil, folinic acid and cisplatin in patients with advanced gastric cancer. Br J Cancer 2000; 83(4): 458–462.
40. Honecker F, Kollmannsberger C, Quietzsch D, Haag C, Schroeder M, Spott C, et al. Phase II study of weekly paclitaxel plus 24-h continuous infusion 5-fluorouracil, folinic acid and 3-weekly cisplatin for the treatment of patients with advanced gastric cancer. Anticancer Drugs 2002; 13(5): 497–503.
41. Kang HJ, Kim TW, Chang HM, Ryu MH, Yook JH, Oh ST, et al. A phase II study of paclitaxel and capecitabine combination chemotherapy in patients with advanced gastric cancer as a first line therapy (abstract). J Clin Oncol 2004; 22 (suppl 14S):4051.
42. Yeh KH, Lu YS, Hsu CH, Lin JF, Hsu C, Kuo SH, et al. Phase II study of weekly paclitaxel and 24-hour infusion of high-dose 5-fluorouracil and leucovorin in the treatment of recurrent or metastatic gastric cancer. Oncology 2005; 69(1): 88-95
43. Park SH, Lee WK, Chung M, Lee Y, Han SH, Bang SM, et al. Paclitaxel versus docetaxel for advanced gastric cancer: a randomized phase II trial in combination with infusional 5-fluorouracil. Anticancer Drugs 2006; 17(2): 225-229.
44. Cho BC, Kim JH, Kim CB, Sohn JH, Choi HJ, Lee YC, et al. Paclitaxel and leucovorin-modulated infusional 5-fluorouracil combination chemotherapy for metastatic gastric cancer. Oncol Rep 2006; 15(3): 621-627.
45. Chao Y, Li CP, Chao TY, Su WC, Hsieh RK, Wu MF, et al. An open, multi-centre, phase II clinical trial to evaluate the efficacy and safety of paclitaxel, UFT, and leucovorin in patients with advanced gastric cancer. Br J Cancer 2006; 95(2): 159-163.
46. Mochiki E, Ohno T, Kamiyama Y, Aihara R, Haga N, Ojima H, et al. North Kanto Gastric Cancer Study Group. Phase I/II study of S-1 combined with paclitaxel in patients with unresectable and/or recurrent advanced gastric cancer. Br J Cancer 2006; 95(12):1642-1647.
47. Ninomiya M, Kondo K, Matsuo K, Hirabayashi N, Kojima H, Kobayashi M, et al. Japanese Southwest Oncology Group (JASWOG). Multicenter phase II trial of combination chemotherapy with weekly paclitaxel and 5-fluorouracil for the treatment of advanced or recurrent gastric carcinoma. J Chemother 2007; 19(4): 444-450.
48. Yamaguchi K, Nakagawa S, Yabusaki H, Nashimoto A. Combination chemotherapy with 5-fluorouracil, cisplatin, and paclitaxel for pretreated patients with advanced gastric cancer. Anticancer Res 2007; 27(5B): 3535-3539.
49. Kawabata R, Fujiwara Y, Doki Y, Fujita J, Tsukahara Y, Yamasaki M, et al. Phase I/II study of a combination of S-1 and weekly paclitaxel in patients with advanced or recurrent gastric cancer. Oncology 2007; 72(3-4): 219-225.
50. Lee HJ, Cho DY, Park JC, Bae SB, Lee KT, Cho IS, et al. Phase II trial of biweekly paclitaxel plus infusional 5- fluorouracil and leucovorin in patients with advanced or recurrent inoperable gastric cancer. Cancer Chemother Pharmacol 2008 Apr 16. [Epub ahead of print]]
51. Kang HJ, Chang HM, Kim TW, Ryu MH, Sohn HJ, Yook JH, et al. A phase II study of paclitaxel and capecitabine as a first-line combination chemotherapy for advanced gastric cancer. Br J Cancer 2008; 98(2): 316-322.
52. Im CK, Jeung HC, Rha SY, Yoo NC, Noh SH, Roh JK, et al. A phase II study of paclitaxel combined with infusional 5- fluorouracil and low-dose leucovorin for advanced gastric cancer. Cancer Chemother Pharmacol 2008; 61(2): 315-321.
53. Inagaki H, Kojima H, Kato J, Kojima T, FujimitsuY, Matsui T, et al. Effective use of combination chemotherapy with paclitaxel and 5-fluorouracil in a case of non-resectable advanced gastric cancer (in Japanese). Gan To Kagaku Ryoho 2003; 30(12): 1983–1987.
54. Wöhrer SS, Raderer M, Hejna M. Palliative chemotherapy for advanced gastric cancer. Ann Oncol 2004; 15(11): 1585–1595.
55. SchöVski P. New drugs for treatment of gastric cancer. Ann Oncol 2002;13(Suppl 4):13–22.
56. Ajani JA, Fairweather J, Dumas P, Patt YZ, Pazdur R, MansWeld PF. Phase II study of Taxol in patients with advanced gastric carcinoma. Cancer J Sci Am 1998; 4(4): 269–274.
57. Kim JG, Sohn SK, Song HS, Kwon KY, Do YR, Lee KH, et al. Multicenter phase II study of weekly paclitaxel plus cisplatin combination chemotherapy in patients with advanced gastric cancer. Cancer Chemother Pharmacol 2007; 60(6):863–869.
58. Gadgeel SM, Shields AF, Heilbrun LK, Labadidi S, Zalupski M, Chaplen R, et al. Phase II study of paclitaxel and carboplatin in patients with advanced gastric cancer. Am J Clin Oncol 2003; 26(1): 37–41.
Correspondence to:
Dr Daoyuan Wang, MD
AmMed Cancer Center, Ruijin Hospital, Medical School of Shanghai Jiaotong University, No. 197, Rui Jin Er Lu, Shanghai 200025, Republic of China
Telephone: +86-21-29750018; Fax: +86-21-54774985; Email: ghealth2008@gmail.com

