Jin Wu*, Hongfeng Zhou, Jincai Wang, Dan Liu
The Affiliated Tumor Hospital of Harbin Medical University, Harbin, Heilongjiang Province, China, 150081
ABSTRACT
Objective
To investigate the relationship of Cx32 and Cx43 expression with the invasion and metastasis of gastric carcinoma.
Methods
Indirect immunofluorescence and RT-PCR were used to detect the expression of Cx32 and Cx43 protein and mRNA in gastric carcinoma cell lines with different metastatic potentials. Gap junctional intracellular communication (GJIC) function was assessed by scrape-loading dye transfer system.
Results
Cx32 and Cx43 were expressed in 100% & 100% of highly differentiated human gastric cell line (GES-1) and 49%, and 55% of less differentiated gastric adenocarcinoma cell line (N87), respectively, However, Cx43 was only localized in cytoplasm of N87. In contrast, Cx32 and Cx43 were negative in least differentiated adenocarcinoma cell line (BGC-823) with high metastasis potential. The similar results were revealed by RT-PCR. GES-1 had a relatively strong GJIC function as the fluorochrome could be transported to 3-4 lines of cells adjacent to the scrape. N87 and BGC-823 cell had an impaired GJIC function since the fluorochrome was restricted within one line of adjacent cells.
Conclusion
Cx32 and Cx43 gene expression and gap junction are vital factors affecting metastatic potentials of gastric carcinoma.
Keywords
Junctional Protein; Gap Junctional Intercellular Communication; Gastric Carcinoma; Metastasis
1.Introduction
Gastric cancer is one of the commonest malignancies threatening human health, and its incidence is increasing in China in recent years. Invasion and metastasis involving a series of interaction between tumor cells and host, are general features of malignant tumors and, also responsible for poor therapeutic response and subsequent death. Among factors influencing the metastatic potential of malignant tumors, Gap Junctional Intercellular Communication (GJIC) is an important one. Gap junction channels are composed of two hexagonal arrays of six subunits connexins (Cx). GJIC plays a critical role in a variety of cell and tissue functions, including regulation of growth, differentiation, and development signaling [1-3], and these functions are closely related with cancer cell invasion and metastasis.
Previous studies demonstrated that Cx32 and Cx43 were expressed in tissues of gastric cancer; however, its role in cell lines is rarely studied. In this study, we therefore used immunohistochemistry, indirect immunofluorescence, RT-PCR, and scrape-loading dye transfer system to assess the expression and function of Cx32, Cx43 in different gastric cancer cell lines in order to explore whether there was consistency for their expressions in tissue and cell, meanwhile analyze influence of their expressions on invasion and metastasis potentials of gastric cancer.
2.Materials and methods
2.1 Materials
Cell Lines
Cell lines GES-1 (Simian virus 40 transformed gastric mucosal epithelial cells), N87 (gastric carcinoma cells with high differentiation), BGC-823 (gastric carcinoma cells with low differentiation) were courteously provided by Beijing Institute of Cancer Research, Ruijin Hospital, and Harbin Institute of Oncology, respectively. GES-1 was cultured in DMEM medium (GIBCO, USA) containing 150 mL/L (15%) sterile fetal bovine serum (FBS) and 1% penicillin-streptomycin in a humidified 37°C incubator and 5 % CO2 atmosphere. N87 and BGC-823 were cultured in RPMI 1640 medium (GIBCO, USA) with the same conditions as GES-1.
Tissue Sampling
58 gastric adenocarcinoma tissue samples were obtained from the Third Hospital of Harbin Medical University. Among them, 20 samples were categorized into intermediate-high grade, and 30 samples into low-grade. All patients did not receive any chemotherapeutic treatments prior to surgery.
Antibodies and Antisera
Antibodies and antisera used in this study included rabbit anti-human polyclonal anti-Cx43 (Zymed Laboratories, USA), goat anti-human polyclonal anti-Cx32 (Santa Cruz Biotechnologies, USA), 488nm goat anti-rabbit IgG (Invitrogen, USA), FITC rabbit anti-goat IgG (Zhongshan, Peking) and rabbit and goat SP kit (Zhongshan, Peking).
2.2 Methods
Indirect Immunofluorescence
Immunofluorescence staining for Cx43 and Cx32 was performed on gastric cancer cells grown in normal or high-glucose medium. Briefly, 5mm×x 5mm round coverslips were placed in 24-well plate, 4×104, 6×104, 10×104 GES-1, N87 and BGC-823 cells were seeded and cultured until 70% cell confluence, then glass coverslips were harvested and cells on coverslips fixed in ice-cold acetone for 15 minutes at -20°C. After PBS washing, cells were blocked with 2% bovine serum albumin in PBS for 15 minutes. Thereafter, cells were incubated with rabbit polyclonal anti-Cx43 and goat polyclonal anti-Cx32 in a moist chamber at 4°C overnight. After PBS washing for three times, cells were incubated with 488nm goat anti-rabbit IgG and FITC rabbit anti-goat IgG for detecting Cx43 and Cx32 protein, respectively. After washing three times, the coverslips were mounted with antifade medium and observed under fluorescence microscope (Diaphot-Nikon, Tokyo, Japan, with Spot software; Diagnostic Instruments, Sterling Heights, MI). Two negative controls were performed in parallel where either the first and second antibodies or the first antibody was not added.
Scrape-Loading Dye Transfer Assay
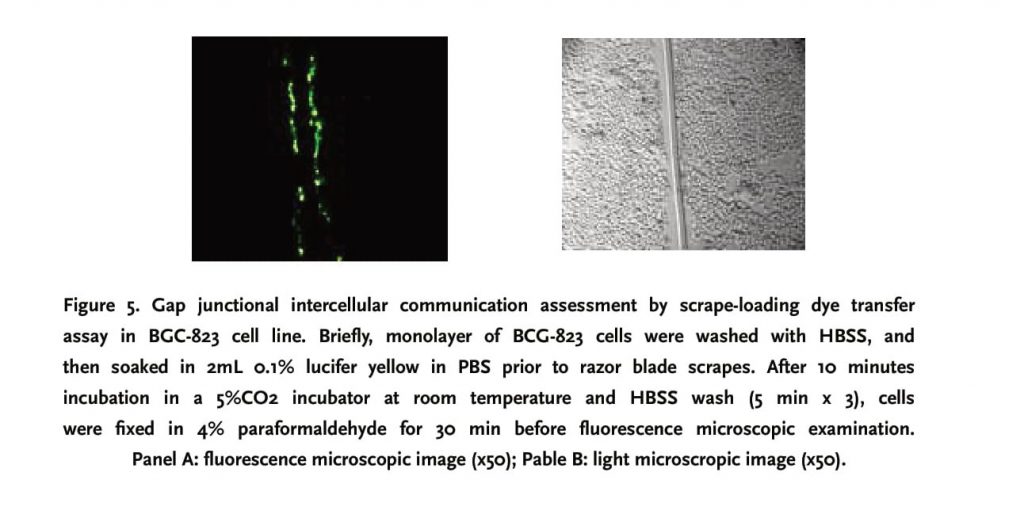
Scrape-loading dye transfer assay is extensively recognized as a useful tool to assess gap junctional function [5, 12]. GES-1, N87 and BGC-823 cell lines were seeded on uncoated 60mm culture dish till 100% confluence. Prior to scraping, cells were washed with HBSS for 5 minutes x thrice, and 2mL 0.1% lucifer yellow diluted in PBS was then added. The cell monolayer was immediately scraped with a razor blade applied with downward force only and incubated in a 5% CO2 incubator for 10 minutes. After HBSS wash (5 min x 3), cells were fixed in 4% paraformaldehyde for 30 min, followed by PBS wash three times. Thereafter, dye-stained cells surrounding scrapes were counted in 10 random low-power fields under fluorescence microscope in order to assess gap junctional intercellular communication.
RT-PCR
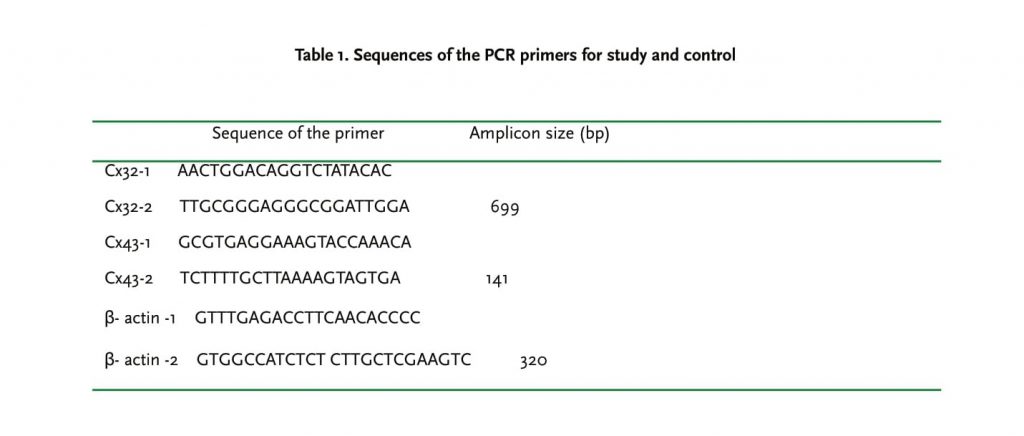
RNA was extracted from three different cell lines in accordance with kit instructions (Promega, USA). ββ- actin was used as internal control. RT-PCR was performed in a 50 μl system of 1 μg RNA, 200 units of reverse transcriptase, 2.5 μmol random hexamers, 1 mmol/L of each dNTPs, 5 mmol/L MgCl2, PCR buffer, and RNase inhibitor at following conditions: 5 min at 94°C for conditioning, 30 sec at 94°C for denaturation, 40s at 59°C for annealing, and 30s at 72°C for extension for 30 cycles, and 7 min at 72°C for last extension. To prevent quantitative inaccuracies deriving from competitive effects and different efficiencies and ranges of amplification of two cDNAs, Cx43 and β-actin cDNAs generated in the same RT reaction were amplified in separate tubes containing increasing volumes of the RT reaction (1, 2, and 4 μl) to document amplification in the linear region for each cDNA. 5 μl PCR amplification products were loaded in 2% agarose gel for electrophoresis. DNA bands on gel were photographed by WD29403 extra violet camera and analyzed by Total-Lab software. The sequences of PCR primers were tabulated in Table 1.
Statistical Analysis
All data were expressed in mean+ SD . Comparisons among groups were conducted with X2 tests. A p value less than 0.05 was considered statistically significant.
3. Results
3.1 Expression and Localization of Cx43 and Cx32 in Gastric Carcinoma Cell Lines
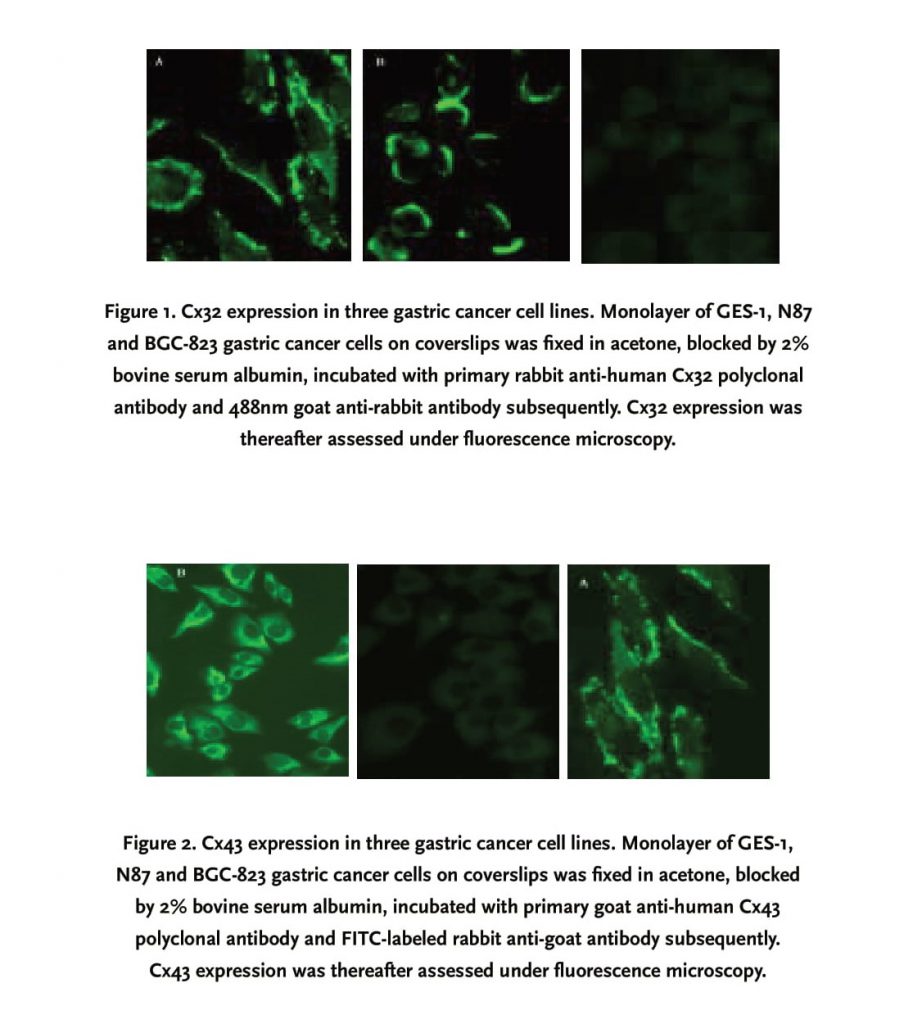
Distribution and relative quantity of Cx43 and Cx32 expression in gastric carcinoma cells were determined by immunofluorescence microscopy as previously mentioned. Cx32 was detected in both GES-1 and N87 cell lines, located mainly on cell membrane in a “dot-line” or linear pattern (Figure 1), but absent in BGC-823 cell line; Cx43 was also positive in GES-1 and N87 cell lines; however, its expression in N87 cell line was diffuse mainly in cytoplasm, other than linear and dotted distribution in GES-1 cell line (Figure 2). Similarly, Cx43 was not minimally visible in the cytoplasm of BGC-823 cell line.
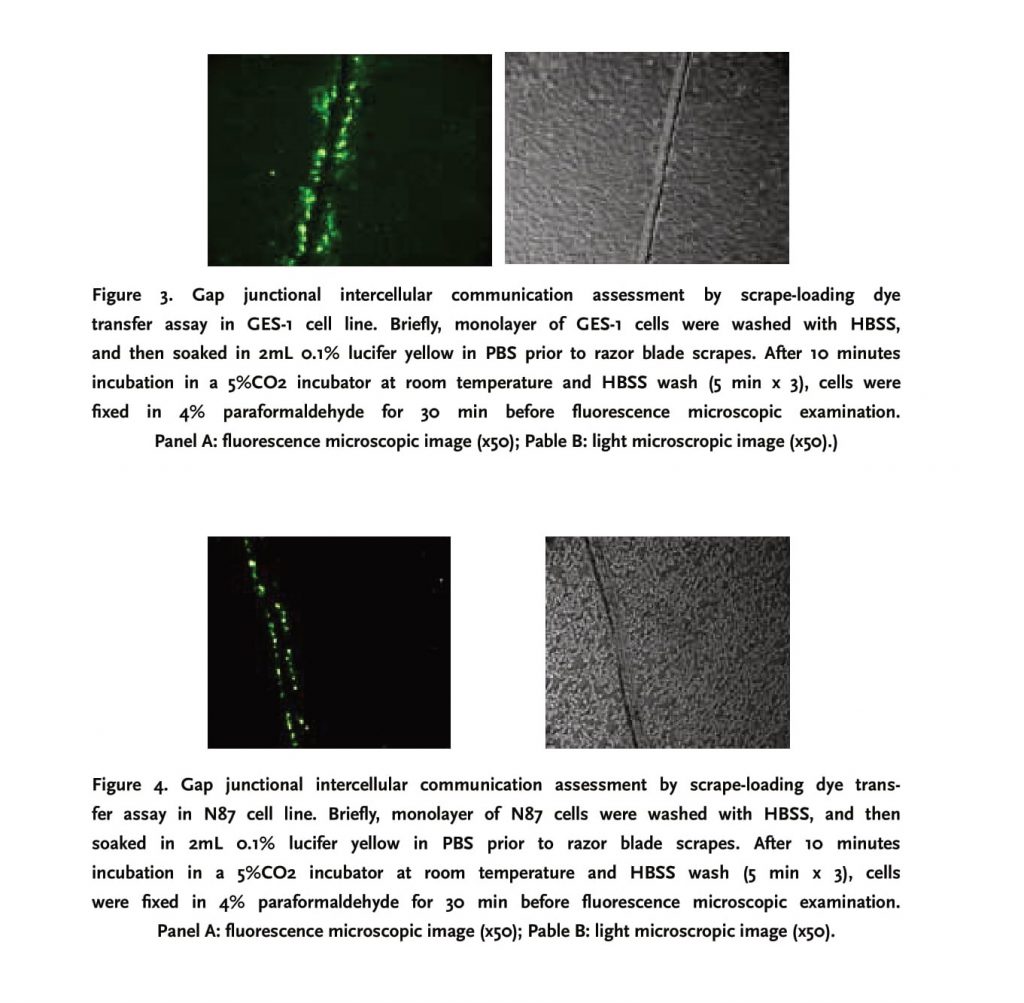
3.2 N87 and BGC-823 lack gap junctional intercellular communication (GJIC), as measured by scrape-loading
Compared to N87 and BGC-823, GES-1 had a relatively strong GIJC function, fluorochrome could be transferred to 3-4 cell lines cells on both sides of the scrape, with a gradually attenuating fluorescent strength from the center to periphery. N87 and BGC-823 cell lines did not exhibit a functional GIJC since fluorochrome was restricted within a single-line cells along the scrape (Figures 3, 4 and 5)
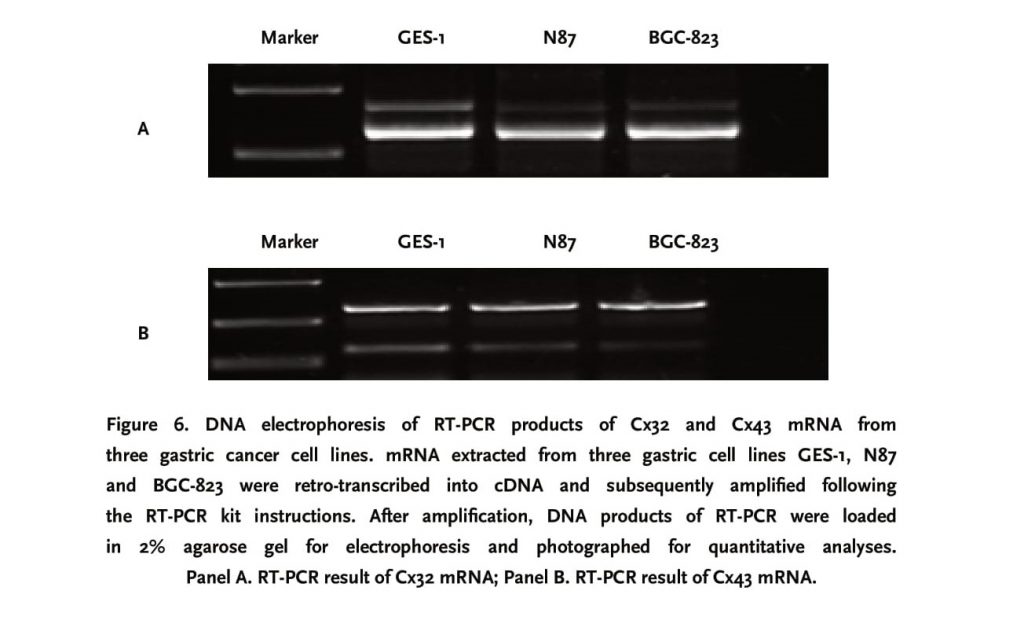
3.3 Cx32 and Cx43 mRNA was strongly expressed in GES-1 and N87 cell lines
RT-PCR results revealed that Cx32 and Cx43 mRNA were all expressed in GES-1, N87 and BGC-823 cell lines. Among three cell lines, the expression level of Cx32 and Cx43 mRNAs, after normalized for internal control β-actin, was highest in GES-1 cell line, and weakest in BGC-823 cell line.
4. Discussion
Human connexin (Cx) genes belong to a homogenous membrane protein family, which has approximately 20 different isomers expressed extensively in different cell types and tissues of mammalians. Gap junction is involved in chemical and electric coupling between cells; therefore, it plays a critical role in material exchange, intercellular communication, and subsequent cell proliferation and differentiation [1-3]. The cardinal feature of malignant tumors is their capability of metastasis; the latter is correlated with the extent of tumor cell differentiation. Till now, a large body of evidence shows that there is a negative correlation of Cx gene expression and gap junction function with malignant extent of tumors. For example, Cx43 knock-out mice have a high incidence of malignancies and expression of Cx genes also decreases in a variety of human cancer cell lines and tissues including adrenocortical carcinoma cell line H295 and hepatocarcinoma tissue [4,5,6,7]. Furthermore, Cx43 mRNA level was significantly downregulated in breast cancer, and recovery of Cx43 expression could reverse the malignant phenotype of the breast cancer in vitro [8]. The inhibitive effect of gap junction is also consolidated by a study where up-regulation of Cx43 and GJIC by Coleusin Factor is associated with growth inhibition in rat osteosarcoma UMR 106 cells [9].
There is a paucity of literatures regarding the gap junction expression in gastric carcinomas and its relationship with malignant extent. Now, the results of this study are in accordance with previous studies on other cancer cells and biopsy tissues. In this study, we discovered that Cx32 and Cx43 mRNAs were strongly expressed in GES-1 and N87 cell lines; however, their expressions were weak in BGC-823. In addition, the expression level of Cx genes in above cell lines is negatively related with the extent of malignancy. For example, Cx32 and Cx43 were strongly expressed in 100% of highly differentiated gastric cell line (GES-1) with a dot-line or linear distribution on cell membrane. Though Cx43 and Cx32 were found in less differentiated N87 cell line, there is a tendency of decreased localization on cell membrane, especially for Cx43 which was diffusely localized in cytoplasm. In order to exert their functions, Cx must be intercalated on cell membrane and compose a hexagonal array of gap junction; therefore, intracellular distribution of Cx43 and Cx32 is not functional as there was a compromised fluorochrome diffusion in a scrape-loading dye transfer system in this study. In the least differentiated BGC-823 cell line, there was weak or no expression of Cx43 and Cx32 genes as well as no functional gap junctional intercellular communication.
As afore-mentioned, GJIC is involved in cell proliferation and differentiation. Once GJIC is impaired, intercellular chemical and electric coupling is consequently disrupted so that critical intracellular signaling molecules such as cAMP, kinases, and IP3 can not effectively transfer among cells, leading to deregulation of cell growth and differentiation. If the above process occurred in cancers, cancer cells would exhibit an ability of immune evasion, high extent of malignancy and strong potential of metastasis. In this study, the complete loss of Cx32 expression and function was correlated with low differentiation and high metastatic ability of gastric adenocarcinoma cell line BGC-823.
There is an interesting finding in this study that Cx was localized in the cytoplasm of gastric cancer cell line N87 other than membrane distribution in normal gastric cells and highly differentiated gastric cell line GES-1. Three potential mechanisms might explain the abnormal localization of Cx in malignant N87 cells: 1) Cx gene in cancer cells could be mutated so that Cx could not be inserted into cell membrane; however, no evidence reveals that there are any mutations in Cx genes; 2) Defects in Cx transcription and post-transcription modification such as phosphorylation; 3) Intracellular factors responsible for Cx transportation might be defective. The transportation of Cx protein from cytoplasm to membrane involves cadherin; however, it remains unclear whether cadherin is abnormal in malignant cell lines.
In conclusion, the current study demonstrates that Cx expression is related to the malignant extent of gastric carcinoma cells as Cx gene expression and functionality decreased with the increment of metastatic potential of tumor cells. Transfection of Cx genes into malignant cells might reverse their malignant phenotype and subsequently inhibit the progression of malignancies, providing a new strategy for the treatment of cancers [10]. However, more studies are warranted to apply this gene therapeutic strategy as a new adjunctive therapy of malignancies.
References
1. Yamasaki H, Krutovskikh V, Mesnil M, Tanaka T, Zaidan-Dagli ML, Role of connexin (gap junction) genes in cell growth control and carcinogenesis. C R Acad Sci III 1999;322(2-3):151-159.
2. Revel, J.P. Karnovsky, M.J. Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. J Cell Biol., 1967; 33: C7-C12.
3. Daniel A. Goodenough, David L. Paul. Beyond the gap: functions of unpaired connexon channels. Nature Reviews Molecular Cell Biology. 2003; 4:285-295.
4. Ma Xiangdong, Sui Yanfang, Wang Wenliang. Expression of gap junction genes connexin 32 and 43 in human hepatocellular carcinoma. Journal of the Fourth Military Medical University. 1999; 20 (7): 593-59
5. Murray SA. Davis K, Fishman LM. Alp hal connexion43 gap junction are decreases in human adrenocortical tumors. J Clin Endocrinol metab 2000; 85:890-895.
6. Geng S, Sun B, Liu S, Wang J. Up-regulation of connexin 43 and gap junctional intercellular communication by Coleusin Factor is associated with growth inhibition in rat osteosarcoma UMR106 cell. Cell Biol Int. 2007; 31(11):1420-7.
7. Sato H, Fukumoto K, Wada S, Hagiwara H et al. Enhancing effect of connexin 32 gene on vinorelbine induced cytotoxicity in A549 lung adenocarcinoma cells. Cancer Chemotherapy Pharmacology. 2007; 60(3):449-57.
8. Saunders MM, seraj MJ, Li Z. Breast cancer metastatic potential correlates with a breakdown in homospecific and hererospecific gap junctional intercellular communication. Cancer Res,2001;61:1765-1767.
9. Sato H, Fukumoto K, Hada S, Hagiwara H et al. Enhancing effect of connexin 32 gene on vinorelbine induced cytotoxicity in A549 lung adenocarcinoma cells. Cancer Chemotherapy Pharmacology. 2007; 60(3):449-57.
10. Pointis G, Fiorini C, Gilleron J, Carette D, Segretain D. Connexins as precocious markers and molecular targets for chemical and pharmacological agents in carcinogenesis Curr Med Chem. 2007;14(21):2288 303.
Corresponding author:
Jin Wu
Tel: (86)045186298730,
Fax: (86)045186298730,
Email: w.u_jin@163.com