Dong Wang, Baochang Cai, Guangming Yang,
Ying Gao, Hao Cai, Junsong Li, Xiao Liu
ABSTRACT
The medicinal plants of Oxytropis species, which contain greatly abundant active compounds and exert prominently effective biological activities, have been widely used in Tibetan and Mongolian medicine. The antitumor activities of Oxytropis have been extensively investigated in the past three decades. The relationships between the antitumor activities and the chemical constituents as well as the structures have been also well reported. Swainsonine, a potent anti-tumor agent, has been studied in clinical trials. The purpose of the mini review is to summarize the available information on the antitumor activities of more than 20 species of commonly used medicinal plants of Oxytropis and to reflect the current status of this research field with a view for the future direction as well as to provide a scientific basis for the further development and utilization of these plants.
KEYWORDS Oxytropis; Anti-tumor activity; Swainsonine, 2’,4’-Dihydroxy chalcone
1 Introduction
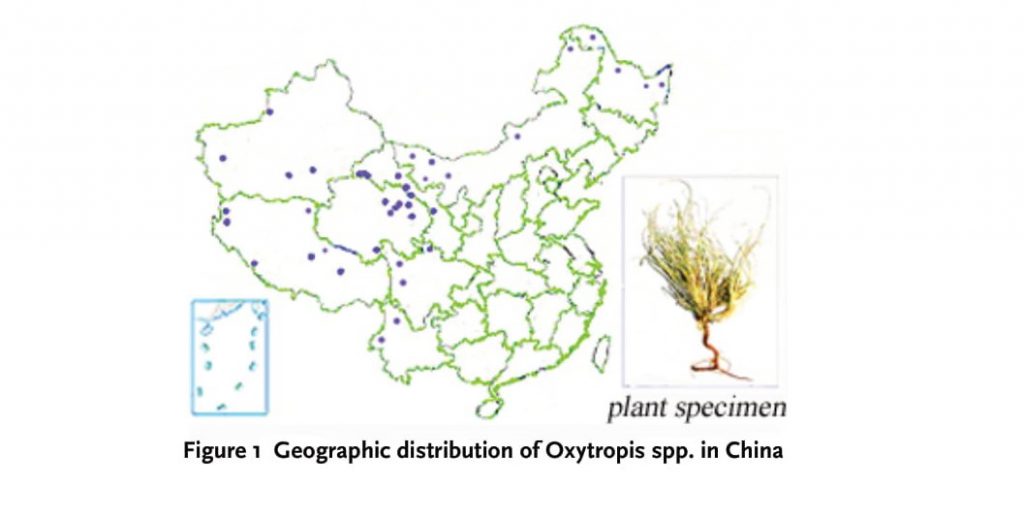
It is well established that plants have been a useful source of clinically relevant antitumor compounds. Indeed, worldwide efforts were made to discover new anticancer agents from plants, which would prevent, slow and/or reverse the cancer induction and its subsequent development. There are many approaches for the selection of plants that may contain biologically active compounds. One of the approaches used is the selection of plants based on the prior information on the folklore medicinal use of plants [1]. Oxytropis genus belongs to the Leguminosae family (subfamily Papilionoidea), with about over 350 species [2], which is distributed practically all over the world and comprises perennial herbs and subshrubs or shrubs growing in the high latitude zones of the world, especially in Russia, America, China, and Canada. In China, 150 species of the plants mainly locate in Tibet and Xinjiang autonomous regions and also distribute in the southwest of the Qinghai-Tibet Plateau and Hengduan mountains regions (Seen Figure 1). The medicinal plants of Oxytropis, widely used in Tibetan and Mongolian medicine, contain greatly abundant active compounds, such as flavonoids, triterpenoid saponins and alkaloids, which possess prominently effective biological activities. They can disinfect and detoxicate, promote granulation and recover sores, also has antitumor, hemostasis [3], anti-inflammatory [4], anti-infective and antinociceptive effects [5]. The purpose of this paper is to sum up and summarize the anti-tumor activities of medicinal plants of Oxytropis and to reflect the current status of this research area with a view for future direction as well as to provide a scientific basis for the further development and utilization of these plants.
2 Anti-tumor activities
Many drugs are derived from active extracts and/or molecules isolated from plants or micro-organisms. Nevertheless, a lot of species have not yet been studied; they constitute an important source of new structures that could possess interesting pharmacological activities and/or mechanisms of action. As far as we know, the anti-tumor activities of Oxytropis have been extensively investigated in the past three decades. The aqueous ethanol extracts, alkaloid fractions and flavonoid compounds showed cytotoxic activity in murine and human cancer cell lines, such as S-180, H-22, ECA-109, SCG-7901, HT-29, MDAY-D2, MGC-803 cells. Swainsonine and 2’,4’-dihydroxy chalcone, the principal anti-tumor active constituents, were reported on the biological activities, recently.
2.1 Aqueous and ethanol extracts
The aqueous and ethanol extracts of O. ochrocephala and O. glabra were proven to have a good inhibition on mice bearing S-180 and H-22 [6, 7]. The same results were obtained by Zhang SX et al [8], they found the ethanol extracts of O. kansuensis can significantly inhibit mice S-37 and H-22 cells in vivo with the inhibition of 39.88% and 51.02%, respectively. As a matter of fact, many compositions including alkaloids, flavonoids, saponins, polysaccharides and organic acids were at least in part attributed to anti-tumor activity of aqueous and ethanol extracts.
2.2 Alkaloid fractions
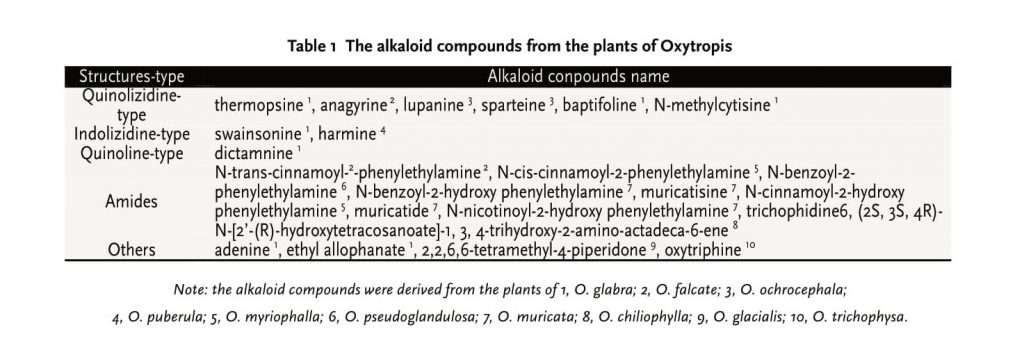
As we all know, Oxytropis species have long been thought to be a locoweed, which can cause significant livestock poisoning and economic loss all over the world [9, 10]. However, so many plants of Oxytropis have been utilized as the medicinal herbs for the various purposes in traditional Tibetan and Mongolian medicine in China for hundreds of years. Alkaloids are one of the major compositions from Oxytropis. It is also considered to be the major toxic substance as well as bioactive substance for many pharmacologic actions, such as anti-tumor and immunization. To explore the clinical value and change “Locoweed” into a useful “Medicinal plant” in the future, many domestic and foreign researchers isolated 23 alkaloid compounds from the plants of Oxytropis. (Seen in Table 1)
Wu D et al [11] screened anti-tumor activities of different fractions, such as ethyl acetate, water, alkaloid, and non-alkaloid fractions, extracted from O. kansuensis in mice bearing S-180 and H-22 in vivo study. The results showed alkaloid fraction had a significant anti-tumor effect (Inhibition (%): 43.57%-58.57%, P<0.01). And to some extent, the ethyl acetate fraction (Inhibition (%): 20.35%-48.67%, P<0.05) and the water fraction (Inhibition (%): 38.05%-41.43%, P<0.05) in part had an effect on the mice, which were both less potent than that of the alkaloid fraction. Liu M et al [12] also found O. kansuensis alkaloid fraction had the same effect on inhibition of the murine ascites sarcoma cell line S-180. The results indicated O. kansuensis alkaloid fraction can inhibit tumor and improve immunity.
The further research has been done by Long L and Li Q [13]. They concluded that O. ochrocephala alkaloid fraction (25 and 50 mg/kg body wt., p.o.) can inhibit H-22 hepatocellular carcinoma growth in mice to various extent in a dose dependent manner (48.5% and 57.7%, respectively, P<0.01). A possible mechanism was suggested that the expression of PCNA and mutant p53 protein in mice bearing H-22 hepatocellular carcinoma was suppressed by alkaloid fraction (p<0.05). Antitumor effect may be associated with the expression inhibition of PCNA and mutant p53 protein.
2.3 Swainsonine
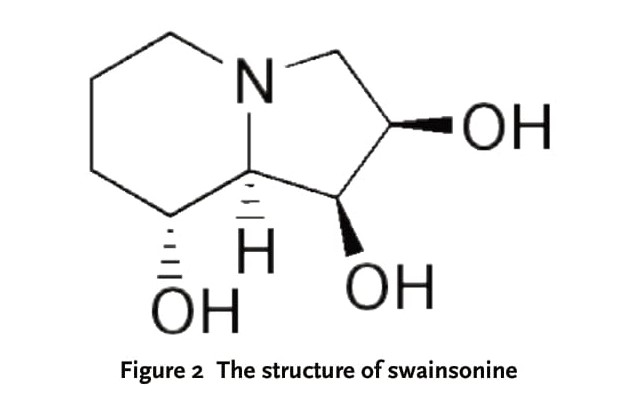
Swainsonine (SW, Seen in Figure 2) is an indolizidine alkaloid, which has high content in the plants of Oxytropis. It has been isolated and derived from the plants of O. falcate, O. glabra, O. kansuensis, and O. ochrocephala, O. glacialis, respectively. Recently, identified molecular targets for new anticancer agents include inducers of cell differentiation, cell cycle arrest, and apoptosis, as well as signaling pathways for growth factors and cytokines. Another novel mechanism of action is presented by the ubiquitous intracellular glycoprotein glycosylation pathway. This complex process, concerned with the addition of sugars onto newly synthesized proteins, occurs in the lumen of the rough endoplasmic reticulum and in the Golgi. SW, a Golgi alpha-mannosidase II inhibitor, is the first to have been selected for clinical trial based on its anticancer activity, bioavailability, and low toxicity in mice [14]. In the first phase I study, 19 patients with both solid tumor and hematological malignancies were given a total of 31 courses. Hepatotoxicity, particularly in patients with liver metastases, was the dose-limiting toxicity. SW produced minimal toxicity when administered i.v. to cancer patients at dosages that inhibit both Golgi alpha-mannosidase II and lysosomal alpha-mannosidases. Detection of hepatic metastases or liver enzyme abnormalities prior to treatment predicted for more significant toxicity [15].
Kino T et al [16] examined SW had potential as an immunomodulator for the treatment of immunocompromised hosts. It could restore the capacities of the immunodeficient mice to produce antibody against sheep red blood cells and inhibit completely the growth of sarcoma ascites tumor S-180 in mice, also reduced lung metastases of B-16 melanoma in mice. Grzegorzewski K et al [17] reported SW was effective in activating peritoneal macrophages to cytotoxicity against tumor cells. Stimulation of tumoricidal activity of macrophages was associated with increased secretion of interleukin-1 (IL-1) and expression of the major histocompatibility complex (MHC) antigen on the cell surface. The anti-tumor activity of SW was due at least in part to immune modulation involving effector cells.
Klein JL et al [18] reported that SW reduced the bone marrow toxicity and protected C57BL/6 mice bearing melanoma-derived tumors from cyclophosphamide induced toxicity as well as human myeloid progenitor cells from AZT (a myelosuppressive agent) toxicity. These results were similar to the research done by Oredipe OA et al [19] on the ability of SW to confer protection against the cytotoxic effects of both cell cycle-specific and cell cycle-nonspecific cytotoxic anticancer agents.
Additionally, lots of experiments in vitro indicated SW isolated from plants could inhibit and eliminate many tumor cells, such as ECA-109, SCG-7901, HT-29, MDAY-D2. Another report found a fungal endophyte had been implicated in the synthesis of Swainsonine in Oxytropis and Astragalus species, commonly known as locoweeds. Li Z et al [20] established cancer model by transplanting Hepatoma 22 cells to rats and poured the secondary metabolites of this fungal endophyte into the stomach of the rats. The TEM method was used to observe pathology changes, and the secondary metabolites had a similar function with SW on inhibiting the growth and metastasis of Hepatoma 22 cells.
SW has generated interest in its potential use as an anticancer agent with reports that (i) inhibits tumor growth and metastasis, (ii) augments natural killer (NK) and macrophage-mediated tumor cell killing, and (iii) stimulates bone marrow cell proliferation. The antineoplastic activity of Swainsonine can be explained at least in part by augmentation of immune effector mechanisms [21]. The protective effects of SW on murine survival and bone marrow proliferation may be useful as an adjuvant and offer promise in future intensive human chemotherapy programs, allowing increased dosage and/or frequency of administration of cytotoxic agents without increasing toxic effects in bone marrow.
2.4 Flavonoid compounds
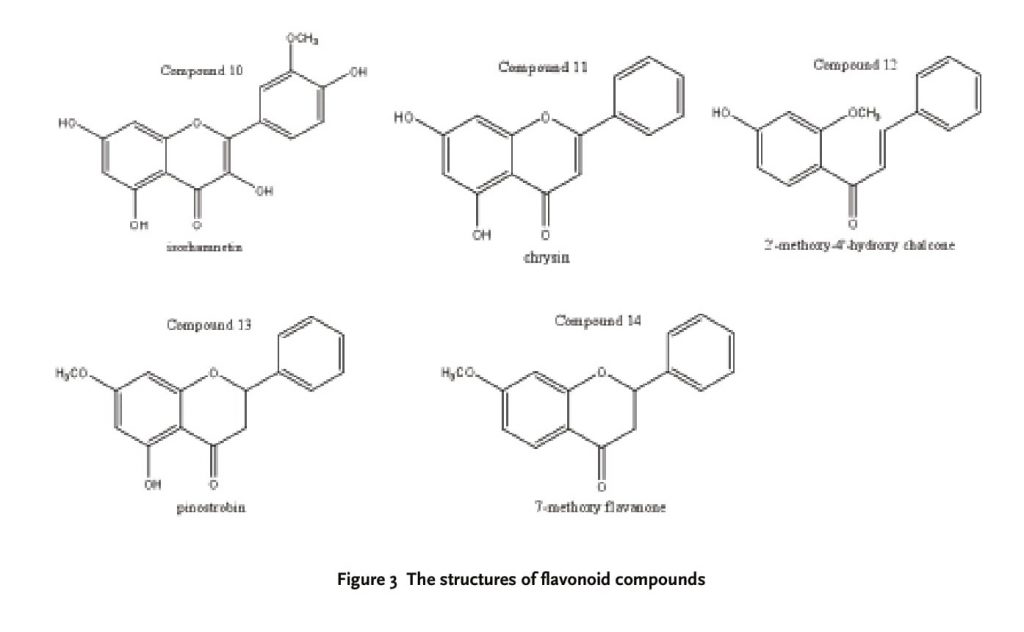
Flavonoid compounds are the major chemical constituents in the plants of Oxytropis. So far, more than 81 flavonoid compounds and derivatives have been isolated and determined from this genus. The flavonoid compounds are of following: 17 flavones and flavone glycosides; 6 flavones; 50 flavonols and flavonol glycosides; 6 chalcones and dihydrochalcones; 1 isoflavone and 1 isoflavane.
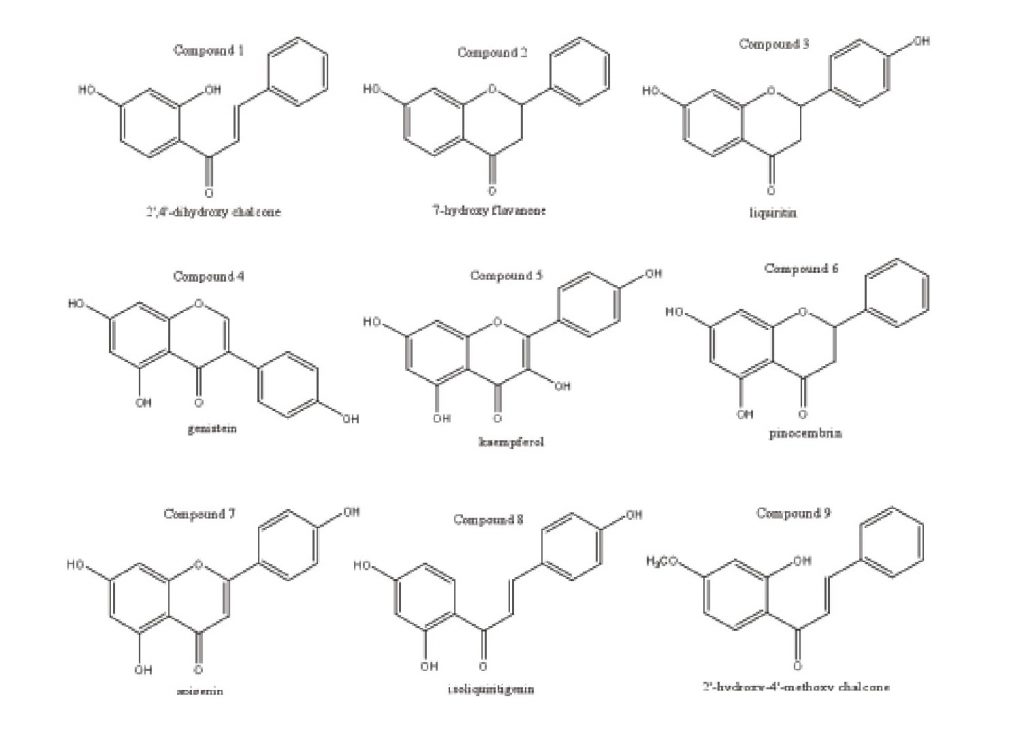
Our previous report [22] elucidated the structures of 13 flavonoid compounds (Seen in Figure 3) from O. falcata bunge and the cytotoxic activities were tested by methods of MTT. Six human tumor cells (SMMC-7721, Hela, A-549, MGC-803, MDA-MB-231 and LOVO) were used in these in vitro experiments and the inhibition of tumor cells was expressed as anti-tumor activities. At a dose of 20 ug/ml, compounds 1, 4, 5, 8, 9 and 12 had a significant inhibition on the growth of human SMMC-7721 cells, and the inhibition rate (%) was as follow: 68.18%, 66.07%, 56.69%, 66.69%, 45.76% and 62.34%, respectively. At the same dose, the growth of human lung cancer A-549 cells was sensitive to compounds 1, 8, 11 and 12. Their inhibition rates (%) were 91.87%, 85.46%, 54.43% and 58.19%, respectively. Compounds 1, 4, 8 and 9 had a better inhibition rate on human colon cancer LOVO cell growth than other compounds with 94.68%, 64.17%, 90.87% and 52.85%, respectively. Human stomach cancer MGC-803 cells were significant inhibited by compounds 1, 4, 8, 11 and 12 at a dose of 20 ug/ml, and the inhibition rates (%) were 90.79%, 79.83%, 79.38%, 78.27% and 71.51%, respectively. The inhibition rates (89.57%, 69.77%, 74.36%, 92.77%, 77.39% and 80.04%) were related to the anti-human cancer Hela cells’ activities of compounds 1, 2, 4, 8, 11 and 12, respectively. The growth of human breast cancer MDA-MB-231 cells was sensitive to compounds 1, 8, 11, 12 and 13. Their inhibition rates (%) were 57.32%, 53.05%, 67.26%, 52.46% and 51.47%, respectively.
Additionally, Yan HH [23] used modern spectrum data and X-ray diffraction technology to elucidate and identify the structures of the two flavones, 2’-hydroxyl-4’-methoxy chalcone (compound 9) and 7-methoxy flavanone (compound 14) from O. falcata bunge, the SRB method was used to test the anti-tumor activities against the SGC cell. The IC50 of compounds 9 and 14 were 3.61 and 6.11 ug/ml, respectively. The results showed that 2’-hydroxy-4’- methoxy chalcone (compound 9) and 7-methoxy-flavanone (compound 14) had significant cytotoxic activities, and further study is needed to explore the mechanism of action.
2.5 2’,4’-Dihydroxy chalcone
Based on our recent study, flavonoid compounds had a dose-dependent anti-tumor activity. Compound 1 (2’,4’-dihydroxy chalcone) can significantly inhibit the proliferation of human tumor cell lines (SMMC-7721, Hela, A-549, MGC- 803, MDA-MB-231 and LOVO cells) in vitro. Our further study [24] found that 2’,4’-dihydroxy chalcone can inhibit the proliferation of human gastric cancer MGC-803 cells and induce apoptosis in MGC-803 cells in a dose and time-dependent manner. The cytotoxicity was mediated by apoptosis, and flow cytometry analysis indicated an increase in apoptotic cells after treatment with 2’,4’-dihydroxy chalcone. Furthermore, typical apoptotic morphology such as condensed chromatin, irregular nuclei, vacuoles, and dispersed granular material in the nuclear compartment were also observed using a transmission electron microscope.
3 Discussion
The medicinal plants of Oxytropis, which contain greatly abundant active compounds and exert prominently effective biological activities, have been widely used in Tibetan and Mongolian medicine. So far, lots of research have been focused on a few species, O. myriophylla, O. glabra, O. falcata, O. kansuensis, O. ochrocephala. Though the anti-tumor activities of Oxytropis have been extensively investigated in the past three decades, further studies are needed to explore and specify their possible mechanism of anti-tumor action in the future.
Reference
[1] Kamuhabwa A., Nshimo C., de Witte P. Cytotoxicity of some medicinal plant extracts used in Tanzanian traditional medicine. J. Ethnopharmacol. 2000; 70 (2): 143-149.
[2] Zhu XY. Outline of the classified system of the Chinese Leguminosae. Bulletin of Botanical Research. 2004; 24 (1): 20-27.
[3] Dai YP, Yang H, Tong L, et al. The preliminary study on hemostatic effect of Oxytropis falcate Bunge. J. Nanjing University of TCM. 2008; 24 (2): 107-108, 126.
[4] Wang D, Yang H, Tong L, et al. Analgesic and anti-inflammatory activity of Tibetan medicine Oxytropis falcata. Pharmaceutical and Clinical Research. 2008; 16 (2): 90-93.
[5] Cai JY. The introduction of Tibetan medicine Oxytropis falcata on prevention and cure of cold. J. Chinese Folk Medicine. 1998; 34 (5): 27.
[6] Li JK. The present situation and prospect of the studies on locoweed in China. Scientia Agricultura Sinica. 2003; 36(9): 1091-1099.
[7] Zhang SX, Cao RG, Li SJ. Inhibition test of extracts from O. ochrocephala to sarcoma in mice. Chinese J. Veterinary Science and Technology. 1991; 21(2): 32-33.
[8] Zhang SX, Cao RG, Li SJ, et al. Inhibition test of ethanol extracts from O. kansuensis on mice tumor S-37 and H-22. J. Animal Science and Veterinary Medicine. 1992; (2):13-15.
[9] Stegelmeier BL, James LF, Panter KE, et al. Tissue Swainsonine clearance in sheep chronically poisoned with locoweed (Oxytropis sericea). J. Anim. Sci. 1998; 76(4): 1140-1144.
[10] James LF, Panter KE, Broquist HP, et al. Swainsonine induced high mountain disease in calves. J. Veterinary and Human Toxicology. 1991; 33(3): 217-219.
[11] Wu D, Long L, Zhang YM. Screening of anti-tumor components from Oxytropis kansuensis. Chin J. Veterinary Science. 2007; 37(5): 440-443.
[12] Liu M, Wang MM, Liu W, et al. Study on anti-tumor effects and immunity function of Oxytropis kansuensis alkaloid fraction. Modern Medicine J of China. 2008; 10(12): 8-11.
[13] Long L, Li QW. The effect of alkaloid from Oxytropis ochrocephala on growth inhibition and expression of PCNA and p53 in mice bearing H-22 hepatocellular carcinoma. Yakugaku Zasshi. 2005; 125(8): 665-670.
[14] Goss PE, Baker MA, Carver JP, et al. Inhibitors of carbohydrate processing: A new class of anticancer agents. Clin J. Cancer Res. 1995; 1(9): 935-944.
[15] Goss PE, Baptiste J, Fernandes B, et al. A phase I study of Swainsonine in patients with advanced malignancies. J. Cancer Res. 1994; 54(6):1450-1457.
[16] Kino T, Inamura N, Nakahara K, et al. Studies of an immunomodulator, Swainsonine. II. Effect of Swainsonine on mouse immunodeficient system and experimental murine tumor. J. Antibiot, 1985; 38(7): 936-40.
[17] Grzegorzewski K, Newton SA, Akiyama SK, et al. Induction of macrophage tumoricidal activity, major histocompatibility complex class II antigen (Iak) expression, and interleukin-1 production by Swainsonine. J. Cancer Commun. 1989; 1(6): 373-379.
[18] Klein JL, Roberts JD, George MD, et al. Swainsonine protects both murine and human haematopoietic systems from chemotherapeutic toxicity. Br J Cancer. 1999; 80(1-2): 87-95.
[19] Oredipe OA, White SL, Grzegorzewski K, et al. Protective effects of Swainsonine on murine survival and bone marrow proliferation during cytotoxic chemotherapy. J. Natl Cancer Inst. 1991; 83(16): 1149-1156.
[20] Zhang L, Yang MQ. Effects of Secondary Metabolites of Rhizoctonia Leguminicola on Hepatoma 22 cells were observed by TEM. Chin J Agriculture Bulletin. 2005; 21(7): 7-10.
[21] Olden K, Breton P, Grzegorzewski K, et al. The potential importance of Swainsonine in therapy for cancers and immunology. J. Pharmacol Ther. 1991; 50(3): 285-290.
[22] Lou CH, Wang MY, Yang H, et al. The Anti-tumor Activity of Flavonoid Compounds from O. falcata bunge in vitro. J. Nanjing University of TCM. 2009; 25(1): 46-50.
[23] Yan HY. Study on structure and biological activity of two flavones from Oxytropis falcata bunge. J. Baoji University of Arts and Sciences (Natural Science). 2008; 28(2): 120-122, 126.
[24] Lou CH, Wang MY, Yang GM, et al. Preliminary studies on anti-tumor activity of 2’,4’-dihydroxychalcone isolated from Herba Oxytropis in human gastric cancer MGC-803 cells. J. Toxicology in Vitro. 2009; 23(5): 906–910.
Correspondence to:
Baochang Cai,
Ph.D., Professor, Nanjing University of Chinese Medicine
E-mail: bccai@126.com
First author: Dong Wang
Master’s Candidate, College of Pharmacy, Nanjing University of Chinese Medicine
Address: P.O. Box 8, 282 Hanzhong Road, Nanjing University of Chinese Medicine, Nanjing 210029, China.
E-mail: pillarwang@126.com
Tel: +86-25-86798281
Fax: +86-25-86798281

