Phytopharmaceutical Research Summary
Sherman Zhao, MD, PhD
ABSTRCT
An integrative approach for treating cancer should target the multiple intracellular pathways that support tumour development and should minimize normal-tissue toxicity. Many traditional medicine manifests anticancer activities. The present article focuses on Dammarane Sapogenins, a group of compounds with anticancer activity generated from panax ginseng. Preclinical studies conducted by Dr. William Jia’s team at University of British Columbia indicated Dammarane Sapogenins have pleiotropic anti-cancer pharmacological effects: inducing tumor cell differentiation and apoptosis through various signalling pathways, reversing multidrug resistance by inhibiting p-gp and synergistic effects with some chemotherapy agents. Moreover, these benefits were also demonstrated in large amount of cancer patients through a number of pilot clinical studies world-wide. Both preclinical and clinical data confirmed the high safety of Dammarane Sapogenins as medicinal preparations.
KEY WORDS Dammarane Sapogenins; cancer apoptosis; multidrug resistance
Panax ginseng background
Ginseng (Panax ginseng) has been used for centuries in Asian medicine as an adaptogen to promote longevity, a tonic for well-being, and a curative, especially in China, Korea, and Japan [1]. The root, legendary for the uncanny resemblance it sometimes bears to the human body, grows slowly and should be several years old before harvesting. Ginseng is referred to as the lord or king of herbs in ancient China [2]. Its medicinal efficacy was first documented in Shennong Ben Cao Jing early in the 3rd century and it was later summarized by Li Shizhen in Ben Cao Gang Mu and Zhong Yao Zhi (Chinese Materia Medica by People’s Health Publishing House, Beijing, published in 1596 and 1959 respectively) [2, 3]. In the 18th century, the effectiveness of ginseng was recognized in the West, and subsequently, a large number of investigations were conducted on its botany, chemistry, pharmacology and therapeutic applications [4-7]. Ginseng is now one of the most popular herbal medicines used nutraceutically worldwide. The pharmacological and therapeutic effects of ginseng have been demonstrated to affect the central nervous system (CNS), cardiovascular system, endocrine regulation, immune function, metabolism, biomodulation action, anti-stress, and anti-aging [5]. The most prominent constituent of ginseng is saponin glycoside compounds known as ginsenosides. Recent research indicates that most of the pharmacological effects of ginseng are attributed to ginsenosides [8]. In general, the contents of ginsenosides vary widely ranging from 2 to 20% depending on the species, age, and part of ginseng, and even vary with the preservation or extraction method [9-11]. More than 30 ginsenosides have been isolated, and characterized from various Panax species [12, 13]. In terms of their chemical structural characteristics, ginsenosides can be classified into three major categories, namely protopanaxadiols (PPD) (e.g., Rb1, Rb2, Rc, Rd, Rg3, Rh2), protopanaxatriols (PPT) (e.g., Re, Rf, Rg1, Rg2, Rh1) and the oleanolic acid derivatives. Ginsenosides are metabolized into Dammarane Sapogenins by β-glucosidase bacteria in the gastrointestinal tract. All Dammarane Sapogenins share a pharmacologically active tetracyclic triterpene backbone. Figure 1 shows the structure of protopanaxadiol. Experimental studies from the past decades have shown that both the anti-tumor activities and therapeutic effects of ginseng are mainly attributed to Dammarane Sapogenins.
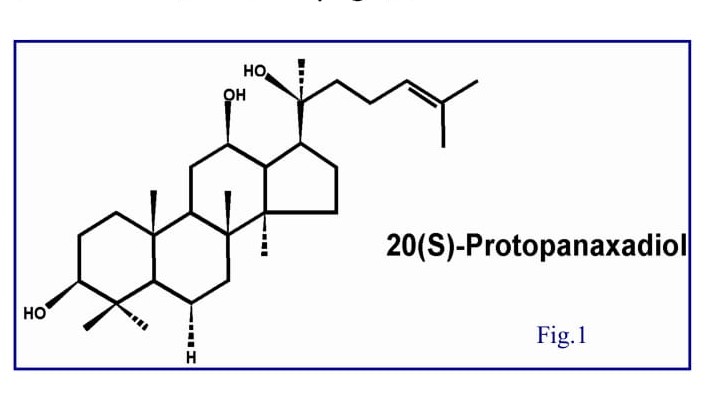
Almost all the studies were concentrated on pre-clinical stages [14-28], few clinical results were reported regarding ginseng products. This situation ascribes to several factors, but cardinally, on one hand, the technology barrier to acquire high concentration of Dammarane Sapogenins limited its medicinal extendibility; on the other hand, very low bioavailability is the most difficult part for this potential drug. With recent advances in technology, the isolation and standardization of these active ingredients for therapeutic use is now possible [29]. Under this advantaged circumstance, Dr. William Jia in collaboration with Pegasus Pharmaceuticals not only developed preparations (in form of soft gel capsule and injection) from ginseng but also conducted in vitro, in vivo, and clinical studies systematically and extensively on the therapeutic effects in cancer. In this review, we focus on the recent advances in the studies of Dammarane Sapogenins on the conquering of cancer which completed by Dr. Jia’s group.
Pharmacological actions of Dammarane Sapogenins In Vitro Studies:
Arresting Cancer cell growth and inducing differentiation
At low concentrations, Dammarane Sapogenins can arrest the cell division at the G1-S transition phase in wild type p53 cancer cells, and at G2 phase in p53 mutated cancer cells.
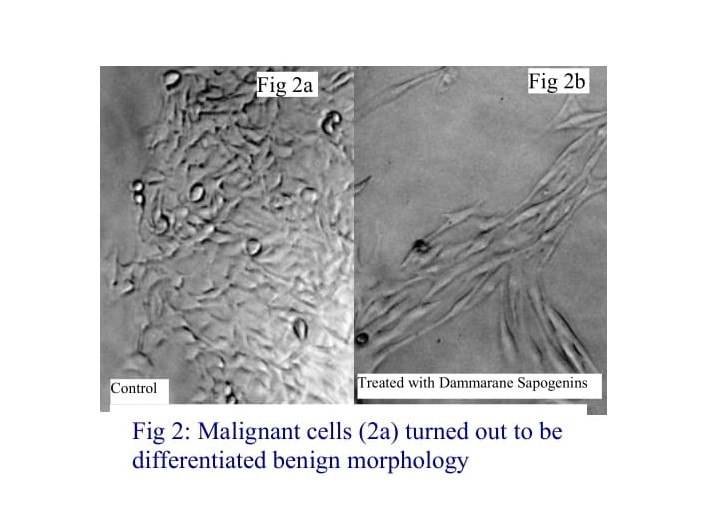
Furthermore, Dammarane Sapogenins can induce malignant cells to differentiate into cells resembling benign cell morphology. Following a 48-hour treatment with Dammarane Sapogenins, the differentiated, spindle-shaped phenotype (Fig. 2b), resemble benign melanocyte morphology (Fig. 2a) was observed in original malignant melanoma cells. Continuously treatment with low concentrations of Dammarane Saponins caused the cells to go apoptosis.
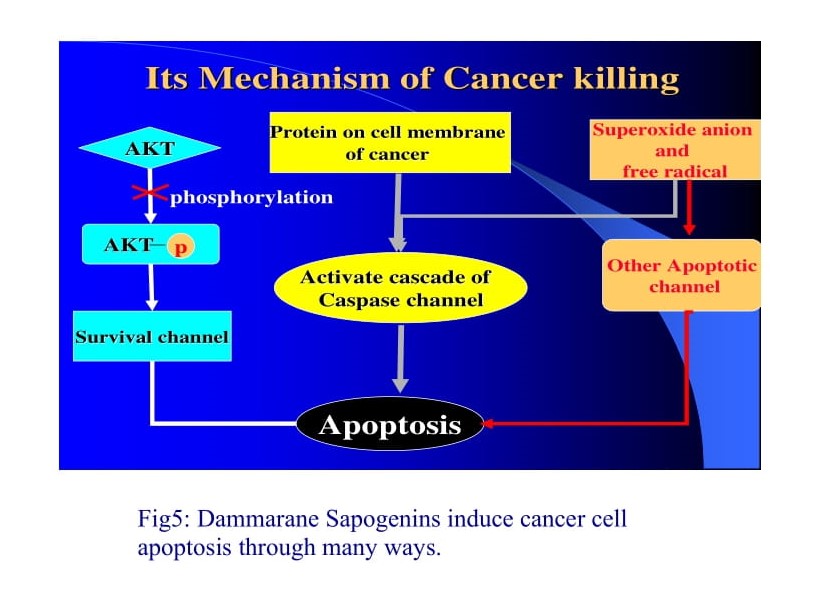
Dammarane Sapogenins induce apoptosis through various signalling cascades [30-32].
Activation of multiple Caspase pathways: Caspase pathway is one of the most important and well documented apoptotic pathways. Dammarane Sapogenins directly activate multiple caspase pathways, including caspases 8 and 9 (the initiators) and caspase 3, 6 and 7 (the executors). At high concentrations, Dammarane Sapogenins directly activate the “executor” caspases to cause apoptosis without the mediation of their up-stream initiators. In this way, Dammarane Sapogenins short cut apoptotic mechanisms, leading to the rapid programmed cell death of cancer cells. Dammarane Sapogenins can also induce apoptosis in certain cancer cells in which the caspase pathways were disabled. This indicates that Dammarane Sapogenins have apoptotic mechanisms that are both caspase dependent and independent.
Inhibition of Akt phosphorylation: Akt is a kinase that inhibits cancer cell apoptosis by opening multiple survival pathways. Akt is activated through the process of phosphorylation. Once activated, not only does Akt inhibits apoptosis, but it also stimulates cell growth. Therefore, one way to inhibit the survival pathway is to down regulate Akt activity. Genetic malfunction in cancer cells, such as mutation in the PTEN gene, results in constitutively activation of the survival pathway. Dr. William Jia and his team at the University of British Columbia discovered that Dammarane Sapogenins effectively inhibit the survival pathway in cancer cells lacking PTEN expression. Western bolting confirmed that the level of Akt phosphorylation is significantly reduced in cancer cells treated with Dammarane Sapogenins. Furthermore, Dammarane Sapogenins arrest Akt biosynthesis in certain cancer cells (MIAPaCa, MCF7, PC3, H460).
Amplification of free radical production in cancer cells, which also trigger apoptosis in cancercells. Recent studies in Dr. Jia’s lab have discoveredanother mechanism for Dammarane Sapogeninsmediated apoptosis. They found that freeradicals within cancer cells are significantly increasedfollowing treatment with DammaraneSapogenins. Most cancer cells have elevatedconcentrations of free radicals following 1 hour ofDammarane Sapogenins administration. Freeradicals can activate various apoptotic pathwaysthrough caspases dependent and caspase independent mechanisms.
Effectively Inhibiting Cancer Cells with Various Mutations
In addition, Dammarane Sapogenins induce apoptosis in cancer cells with different genetic backgrounds. The normal expression of p53 arrests cellular division, which eventually results in apoptosis. However, p53 is prone to mutation. This mutation can cause normal cells to become cancerous. Furthermore, some chemotherapeutic drugs require the normal function of p53 in order to be effective. Therefore, a mutation of p53 may lead to multidrug resistance. PTEN mutations are also common in cancer cells. As previously mentioned, PTEN regulates the survival pathway through the activation of Akt. Once the PTEN gene is lost, cancer cells can become drug resistant through the activation of Akt. Dr. Jia’s studies found that Dammarane Sapogenins exhibit anticancer effects regardless of whether the normal functions of the p53 or PTEN genes have been compromised, this efficacy demonstrated by the induction of apoptosis in SF188 (lacks the p53 gene) cell line and U87 (lost the PTEN gene) cell line.
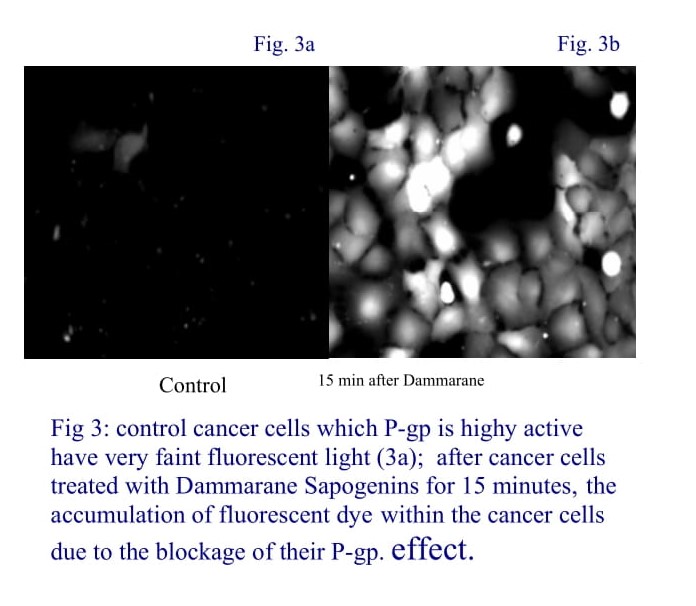
Effects of Dammarane Sapogenins on multidrug resistance
The development of multi-drug resistance (MDR) is a major problem in cancer chemotherapy. Agents that can enhance the accumulation of chemotherapeutic drugs in tumor cells by targeting the MDR proteins is a novel approach to overcome this problem. Dr. Jia’s works revealed that Dammarane Sapogenins could be the one. Several in vitro studies suggest that Dammarane Sapogenin completely reversed P-gp induced drug resistance to Taxol or Doxorubicin on MCF-7adr cells. Dammarane Sapogenins are effective P-gp blocker with a mechanism different from that of verapamil. Given extremely low toxicity of the compound, Dammarane Sapogenin is a potential candidate of chemosensitizer for treatment of multidrug resistant tumors [33-35].
Many other literatures supported that Dammarane Sapogenins could exert chemosensitizing agents [36-39].
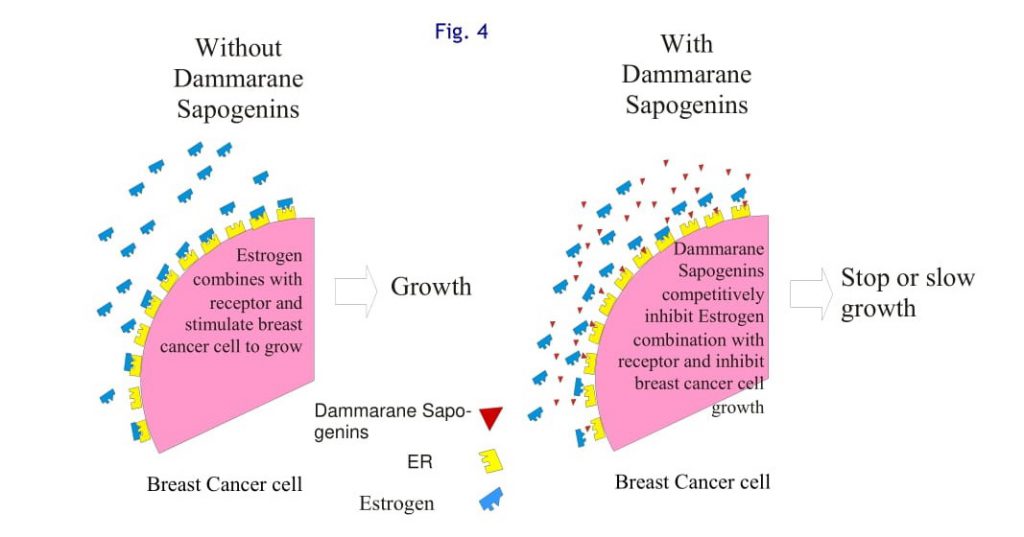
Dammarane Sapogenins is a competitive inhibitor of Estrogen Receptors
Some Dammarane Sapogenins can bind to estrogen receptors, but they have very weak estrogen like actions. In this way, these Dammarane Sapogenins competitively inhibit the growth-stimulating effects of estrogen, as illustrated in Figure 4. This mechanism has clinical significance for breast cancer prevention, treatment, and lasting remission [40]. This effect has been found by other researchers.
Dammarane Sapogenins are Effective in Advanced and Multi-drug Resistant Cancers
The treatment of advanced cancers has yet to be adequately developed. Despite the most innovative standard medical treatment, cancers relapse in the majority cases. Most recurrent cancers are drug resistant. Advanced, multidrug resistant cancers are difficult to control. However, Dammarane Sapogenins have demonstrated efficacy in the treatment of advanced and multidrug resistant cancers. Dr. Jia’s team conducted studies on wild type cell line P388wt and its P-gp over expression pair P388adr. Results indicated that Doxorubicin is effective in killing P388wt, IC50 at 0.064μM. Meanwhile, Doxorubicin is far less effective in killing MDR cancer P388adr, with an IC50 at 20μM, which is over three-hundred times greater than the amount required for P388wt. However, when Dammarane Sapogenins is incubated with P388adr, it turned out to be more sensitive cytotoxic agent than doxorubicin. Thus, it seems that Dammarane Sapogenins is equally effective in causing cancer cell cytotoxicity on drug resistant cancer cells. These results have been confirmed by the clinical application of Dammarane Sapogenins for advanced and MDR cancer patients.
Anti-angiogenic effects of Dammarane Sapogenins
Accumulating evidence demonstrated antiangiogenic activity of Dammarane Sapogenins in different tumor models. In those animal models which have been orthotopically implanted with different tumor cells such as human breast infiltrating duct carcinoma, ovarian carcinoma SKOV3 cells, B16-BL6melanoma cells and colon 26-M3.1 carcinoma, Rg3 or Rb2, was found to inhibit angiogenesis in vivo. A significant reduction of intra- tumoral microvessels density (MVD), VEGF (vascular endothelial growth factor) mRNA and VEGF protein levels in tumor tissues and sera of the tumor-bearing animals was observed [42, 43]. Though these two components of ginseng are ginsenoside, it’s believed they are metabolized to Dammarane Sapogenins by enzymatic reactions, and the form of aglycone is their active type. Similar results were also reported when Dammarane Sapogenins was used in combination with a low dose of cyclophosphamide in bearing mice Lewis lung carcinoma [44]. These data indicate that one of the mechanisms of anticancer effect of Dammarane Sapogenins is probably related to suppression of tumor induced angiogenesis [45]. All these mechanisms in combination with findings by Dr. Jia indicated Dammarane Sapogenins are multi-targeting.
Pharmacological Actions of Dammarane Sapogenins In Vivo:
Dammarane Sapogenins Are Effective in Treating Brain Cancers
Dammarane Sapogenins were studied in brain cancer animal models. Subcutaneous implantation of glioma was allowed to develop to a given size before the administration of Dammarane Sapogenins treatment or placebo. Results are expressed in a Kaplan-Meier survival curve. The entire control group died within 24 days. Meanwhile, 40% of animals in the 25 mg/kg group survived for 40 days, the longest period observed. The survival was dose dependent. Dammarane Sapogenins administration causes a significant reduction in the size of glioma in a subcutaneous xenograft model. Prostate and pancreatic cancer models also demonstrated similar results.
Dammarane Sapogenins Inhibit Breast Cancer in Animal Models, and are synergistic with Tamoxifen
According to the studies conducted by Dr. William Jia at UBC, oral administration of Dammarane Sapogenins is effective in the treatment and prevention of breast cancer both estrogen-dependent and independent. The studies demonstrated that Dammarane Sapogenins have competitive inhibitory effects on estrogen receptor sites, decreasing the estrogen-to-estrogen receptor binding. Decreased estrogen receptor binding leads to a reduction in the growth of estrogen-dependent breast cancer. In a breast cancer animal model, Dammarane Sapogenins completely arrested the growth of estrogen-dependent breast cancer in high estrogen exposure. Furthermore, when used in combination, Dammarane Sapogenins can enhance the effects of Tamoxifen independent of the estrogen dependency. Dammarane Sapogenins have also demonstrated the ability to inhibit the stimulatory effects of estrogen on early-stage breast cancers. Tamoxifen does not possess such an effect. Based on these studies, Dammarane Sapogenins therapy has demonstrated the potential to outperform Tamoxifen in the prevention of breast cancer recurrence and metastasis.
The Pharmacological Actions of Dammarane Sapogenins Intravenous Treatment
Animal studies demonstrated that intravenous administration of Dammarane Sapogenins inhibit the growth murine sarcoma S180, murine melanoma B16, murine Lewis lung cancer, and murine colon cancer colon-26 in a dose dependent manner. The inhibition rate of murine sarcoma S180 and murine melanoma was 36-55% and 32-48% respectively, following 7 consecutive daily intra- venous injections of Dammarane Sapogenins at dosages of 15-60 mg/kg. The inhibition rate of murine Lewis lung cancer and murine colon cancer colon-26 was 36-54% and 31-47% respectively, following 10 consecutive daily intravenous injections of Dammarane Sapogenins at dosages ranging from 15-60 mg/kg. In the model of intestine cancer LOVO implanted in nude mice, high dose (60 mg/kg) caused a 60% inhibition rate following a course of 10 consecutive daily intravenous injections.
A low dose of 15 mg/kg causes a 30% inhibition. A dose of 10 mg/kg of intravenous administration of Dammarane Sapogenins was used in combination with Adriamycin, cisplatin, and paclitaxel in the treatment of murine sarcoma S180. The results revealed that Dammarane Sapogenins enhancing the effects of these drugs.
Intravenous administration of Dammarane Sapogenins has demonstrated an affect on the immune systems in mice bearing Lewis lung cancer. Although low dose did not show obvious evidence of immunomodulation, higher dosages can enhance the growth of the spleen lymph cells and increase the activation of Natural Killer cells.
In summary, pre-clinical studies demonstrate that intravenous Dammarane Sapogenins therapy has strong anticancer activities in many types of xenograft animal models. Furthermore, the results indicate a dose dependence mode. In addition, Dammarane Sapogenins enhance the immune function of the tumor-bearing animals.
Toxicology Studies of Dammarane Sapogenins
1. Toxicology of Orally Administered Dammarane Sapogenins
Acute toxicology studies demonstrated that Dammarane Sapogenins, at dosages (4,000 mg/kg) equivalent to 550 times higher than the human dose do not cause any adverse reactions. Long-term toxicology studies (8 weeks) demonstrated that the long-term use of Dammarane Sapogenins at dosages equivalent to 50 times higher than the human dose do not cause any abnormality in the heart, liver, kidney, and blood counts; nor did these dosages cause damage to the nervous system.
2. Toxicology of Dammarane Sapogenins in Injectable Dosage Form
Hemolysis and pyrogen tests, blood vessel irritation test, allergy tests in Cavia porcellus, all these examinations necessary for injection preparations demonstrated Dammarane Sapogenins’ safety.
Acute toxicity studies obtained LD50’s for injectable Dammarane Sapogenins, LD50 for female mice is 511.28 mg/kg body weight; for male mice it is 494.70 mg/kg. These dosages are 15 times higher than effective clinical doses.
Pharmacokinetics of Dammarane Sapogenins was also investigated. It is found that hard shell capsules with Dammarane Sapogenins powder have the poorest oral absorption. Oral solution has increased absorption. However, the soft gel capsules resulted in the best overall oral absorption. The blood level peaked between 30 and 40 Dammarane Sapogenins minutes. The organ distribution of Dammarane Sapogenins is positively correlated with the dose in animals and showed that Dammarane Sapogenins may be able to cross the blood-brain barrier. These findings demonstrate that Dammarane Sapogenins may be useful in the treatment of malignant brain tumors, whether primary or metastatic.
A New Class of drug: Clinical Studies of Dammarane Sapogenins
Two formulations of Dammarane Sapogenins, soft gel and intravenous injection have been developed. Dammarane Sapogenins preparations are indicated as adjuvant treatment in combination with chemotherapy agents. To date, thousands of advanced cancer patients have benefited from Dammarane Sapogenins therapy, generally these benefits are effectively reduced tumor size, stabilize disease condition, improve life quality and physical conditions, prolong survival time, and prevent recurrence and metastases. With prostate and colorectal cancer patients, the tumor marker CEA and PSA had lowered or stabilized during treatment. In 2005 and 2006, another clinical study was conducted. High doses Dammarane Sapogenins I.V (5000mg ~ 7000mg per day) were used in the treatment of advanced cancers. The overall response rate (including complete and partial responses, and stable disease) for primary lung tumor lesions was over 80- percent. Furthermore, 90-percent of patients saw an increase in their WBC count, which demonstrates bone marrow function restoration from damage because of conventional chemotherapy. When these preparations were in combination with other cytotoxic agents such as Paclitaxel et al, less adverse events and improved outcomes were observed. The study also demonstrated that Dammarane Sapogenins was well tolerated by patients, with very high clinical safety. Most commonly, occurred side effects concerning Dammarane Sapogenins include gastrointestinal discomfort, mild allergic reactions such as rush, redness et al [46, 47].
It is especially noteworthy that Dammarane Sapogenins cross the Blood-Brain Barrier and have been used to treat primary and metastatic brain cancers. Numerous cases showed, as single agent, Dammarane Sapogenins can be administered to patients with metastasis brain cancer, and promising outcomes had been observed (significant shrinkage even disappearance of tumor).
Additional from above mentioned effects, Dammarane Sapogenins have demonstrated Pleiotropic Effects for Cancer Patients:
Analgesic Effect
An analgesic effect lasting between 12 and 57- hours following administration in all 13 patients evaluated in a clinical trial for middle to late-stage primary and metastatic NSCLC. All 13 patients (100%) responded with NRS below 3. The pain relief after administration was obvious, accompanied with improvement of sleep, appetite, and other behavioral benefits. The relief lasted for 2 to 5 days after administration.
Impact on bone marrow functions
Dammarane Sapogenins showed a significant effect on improving the bone marrow function after chemotherapy and/or radiotherapy, especially neutropenia. Some patients who had above normal WBC due to infection saw the count fall back.
Effect on immune system
Dammarane Sapogenins demonstrated impact on immune system. The proportion of TH1/Th2 was reduced by 20, and the average values of IgE (Allergy) and IL-6 were decreased. Suggesting that Dammarane Sapogenins preparations enhanced humoral immune function.
Conclusion:
At present, ginseng is not only used as therapeutics by traditional medical practitioners but is also as medications available in the commercial market. The diversity of highly desirable pharmacological effects of ginseng has intrigued scientists for years. Two Dammarane Sapogenins based ginseng products have been commercially available and large-scale phase II studies are undergoing right now. Although the modulatory and therapeutic effects of ginseng components have been extensively investigated, the actual molecular mechanisms remain largely unknown. Recently, it has been found that ginsenosides can act as functional ligands to activate different cellular receptors including some steroid receptors [48-51]. The interaction between ginsenosides and various nuclear steroid hormone receptors may explain the diverse activities of ginseng, which may eventually lead to further development of ginseng-derived therapeutics for a variety of diseases.
Acknowledgement:
Dr. William Jia’s team at University of British Columbia.
References:
1. Yun TK: Brief introduction of Panax ginseng C.A. Meyer. J Korean Med Sci 2001:3-5.
2. Hu SY: A contribution of our knowledge of ginseng. Am J ChinMed 1977, 5:1-23.
3. Tao HC: Sheng-Nung-Pen-Tsao-Ching Taipei, Taiwan: Chung-Hwa;1955.
4. Liu CX: Introduction on research of ginseng. Information of Traditional Chinese Medicine 1975, 2:9-11.
5. Liu CX: Pharmacology and clinic of active principles of ginseng. Chinese Traditional Herbs and Drugs 1975, 7:57.
6. Liu CX, Xiao PG: Recent advances on ginseng research in China. J Ethnopharmacol 1992, 36:27-38.
7. Jiang JW, Xiao QS: Handbook of Active Constituents of Medicinal Plants Beijing: People’s Health Publishers; 1985:503-516.
8. Wang BX: Progress of pharmacological studies of ginseng. Yaoxue Xuebao 1980, 15:312-320.
9. Huang KC: The Pharmacology of Chinese Herbs Boca Raton, FL: CRCPress; 1999.
10. Shoji J: Recent advances in the chemical studies on ginseng. In Advances in Chinese Medicinal Materials Research Edited by: Chang HMYeung HW, Tso WW, Koo A. Singapore: World Scientific; 1985.
11. Tanaka O, Kasai R, Morita T: Chemistry of ginseng and related plants: recent advances. Abstr Chin Med 1986, 1:130-152.
12. Wang X, Sakuma T, Asafu-Adjaye E, Shiu GK: Determination of ginsenosides in plant extracts from Panax ginseng and Panax Quinquefolius L. by LC/MS/MS. Anal Chem 1999, 71:1579-1584.
13. Yu H, Zhang C, Lu M, Sun F, Fu Y, Jin F: Purification and characterization of new special ginsenosides hydrolyzing multi-glycosides of protopanaxadiol ginsenosides, ginsenosides type I. Chem Pharm Bull (Tokyo) 2007, 55:231-235.
14. Yan-lin Ming, Gang Song, Liang-hua Chen, Zhi-zhong Zheng, Zhong-yan Chen, Gao-liang Ouyang and Qingxuan Tong Anti-proliferation and apoptosis induced by a novel intestinal metabolite of ginseng saponin in human hepatocellular carcinoma cells. Cell Biology International 2007, 31: 1265-73.
15. Carolina Scarpa Carneiro, Frederico Azevedo Costa- Pinto, Ana Paula da Silva, Kátia Cristina Pinello, Tereza Cristina da Silva, Patrícia Matsuzaki, Márcia Kazumi Dr. William Jia’s team at University of British Columbia Nagamine, Silvana Lima Górniak, Mitsue Haraguchi, Gokithi Akisue, et al. Pfaffia paniculata (Brazilian ginseng) methanolic extract reduces angiogenesis in mice. Experimental and Toxicologic Pathology 2007, 58: 427-31.
16. Shinkai K, Akedo H, Mukai M, Imamura F, Isoai A, Kobayashi M, et al. Inhibition of in vitro tumor cell invasion by ginsenoside Rg3. Jpn J Cancer Res 1996, 87:357-62.
17. Popovich DG, Kitts DD. Structure-function relationship exists for ginsenosides in reducing cell proliferation and inducing apoptosis in the human leukemia (THP-1) cell line. Arch Biochem Biophys 2002, 406:1-8.
18. Kim HE, Oh JH, Lee SK, Oh YJ. Ginsenoside RH-2 induces apoptotic cell death in rat C6 glioma via a reactive oxygen- and caspase dependent but Bcl-X(L)-independent pathway. Life Sci 1999, 65: PL33-40.
19. Kim YS, Jin SH, Lee YH, Kim SI, Park JD. Ginsenoside PAN-11 induces apoptosis independently of Bcl-2, Bcl-xL, or Bax in C6Bu-1 cells. Arch Pharm Res 1999, 22: 448-53.
20. Oh M, Choi YH, Choi S, Chung H, Kim K, Kim SI, et al. Anti-proliferating effects of ginsenoside PAN-11 on MCF-7 human breast cancer cells. Int J Oncol 1999, 14:869-75.
21. Nakata H, Kikuchi Y, Tode T, Hirata J, Kita T, Ishii K, et al. Inhibitory effects of ginsenoside PAN-11 on tumor growth in nude mice bearing human ovarian cancer cells. Japan J Cancer Res 1998, 89:733-40.
22. Huong NT, Matsumoto K, Kasai R, Yamasaki K, Watanabe H. In vitro antioxidant activity of Vietnamese ginseng saponin and its components. Biol Pharm Bull 1998, 21: 978-81.
23. Iishi H, Tatsuta M, Baba M, Uehara H, Nakaizumi A, Shinkai K, et al. Inhibition by ginsenoside Rg3 of bombesin-enhanced peritoneal metastasis of intestinal adenocarcinomas induced by azoxymethane in Wistar rats. Clin Exp Metastasis 1997, 15: 603-11.
24. Kikuchi Y, Sasa H, Kita T, Hirata J, Tode T, Nagata I. Inhibition of human ovarian cancer cell proliferation in vitro by ginsenoside PAN-11 and adjuvant effects to cisplatin in vivo. Anticancer Drugs 1991, 2: 63-7.
25. Kim SE, Lee YH, Park JH, Lee SK. Ginsenoside- Rs4, a new type of ginseng saponin concurrently induces apoptosis and selectively elevates protein levels of p53 and p21WAF1 in human hepatoma SK-HEP-1 cells. Eur J Cancer 1999, 35: 507-11.
26. Tereza Cristina da Silva, Ana Paula da Silva, Gokithi Akisue, José Luis Avanzo, Márcia Kazumi Nagamine, Heidge Fukumasu, Patrícia Matsuzaki, Paulo César Raspantini, Mitsue Haraguchi, Silvana Lima Górniak, et al. Inhibitory effects of Pfaffia paniculata (Brazilian ginseng) on preneoplastic and neoplastic lesions in a mouse hepatocarcinogenesis model. Cancer Letters, 2005, 226: 107- 13
27. Seon-Hee Oh and Byung-Hoon Lee. A ginseng saponin metabolite-induced apoptosis in HepG2 cells involves a mitochondria-mediated pathway and its downstream caspase-8 activation and Bid cleavage. Toxicology and Applied Pharmacology 2004, 194: 221-9.
28. Sang-Jun Lee, Won-Gil Ko, Jeong-Hee Kim, Jong-Hwan Sung, Sang-Joo Lee, Chang-Kiu Moon and Byung-Hoon Lee Induction of apoptosis by a novel intestinal metabolite of ginseng saponin via cytochrome c-mediated activation of caspase-3 protease. Biochemical Pharmacology 2000, 60:677-85.
29. Jia W. Post-absorption/metabolism multi compound drug: a new approach to modernization of Chinese herbal medicine. Zhong Xi Yi Jie He Xue Bao 2006, 4:441-6.
30. Jia W, Yan H, Bu X, Liu G. Anticancer pharmacology of careseng, a natural product of ginseng. ASCO Annual Meeting 2003, Abstract No: 3571.
31. Liu GY, Bu X, Yan H, Jia WW. 20Sprotopanaxadiol- induced programmed cell death in glioma cells through caspase-dependent and – independent pathways. J Nat Prod. 2007, 70:259-64.
32. G.Liu, H.Yan, L.Bu, and W.Jia. Aglycon ginsenosides induce apoptosis in cancer cells via activating caspases. AACR 94th Annual Meeting 2003.
33. W. Jia, H. Yan, X. Bu, G. Liu, Y. Zhao. Aglycone Protopanaxadiol, a ginseng saponin inhibits P-glycoprotein and sensitizes chemotherapy drugs on multidrug resistant cancer cells. ASCO Annual Meeting, 2004 Abstract No: 9663.
34. Guoyu Liu, Yan Zhao, Hang Yan, Xuexian Bu, William Jia. 2983 An aglycon ginsenoside induces apoptosis and blocks p-glycoprotein in multidrug resistance cancer cells. 95th AACR Annual Meeting 2004.
35. Jia WW, Bu X, Philips D, Yan H, Liu G, Chen X, Bush JA, Li G. Rh2, a compound extracted from ginseng, hypersensitizes multidrug-resistant tumor cells to chemotherapy. Can J Physiol Pharmacol 2004, 82:43-7.
36. Seung-Whan Kim, Hyog-young Kwon, Dong-Whan Chi, Jai-Heon Shim, Jong-Dae Park, You-Hui Lee, Suhkneung Pyo and Dong-Kwon Rhee Reversal of Pglycoprotein- mediated multidrug resistance by ginsenoside Rg3 Biochemical Pharmacology, 2003, 65: 75-82.
37. Jing Jin, Sanjay Shahi, Hee Kyoung Kang, Hendrik W. van Veen, and Tai-Ping Fan Metabolites of ginsenosides as novel BCRP inhibitors. Biochemical and Biophysical Research Communications 2006, 345: 1308-14.
38. Hasegawa H, Sung JH, Matsumiya S, Uchiyama M, Inouye Y, Kasai R, Yamasaki K: Reversal of daunomycin and vinblastine resistance in multidrug-resistant P388 leukemia in vitro through enhanced cytotoxicity by triterpenoids. Planta Med 1995, 61:409-413.
39. Berek L, Szabo D, Petri IB, Shoyama Y, Lin YH, Molnar J: Effects of naturally occurring glucosides, solasodine glycosides, ginsenosides and parishin derivatives on multidrug resistance of lymphoma cells and leukocyte functions. In Vivo 2001, 15:151-156.
40. Yu Y, Zhao Q, Hang Y, Bu X, Jia W. Antiestrogenic effect of 20S-protopanaxadiol and its synergy with tamoxifen on breast cancer cells. Cancer. 2007, 109: 2374-82.
41. Wings T.Y. Loo, Mary N.B. Cheung and Louis W.C. Chow The inhibitory effect of a herbal formula comprising ginseng and Carthamus tinctorius on breast cancer. Life Sciences2004, 76: 191-200.
42. Mochizuki M, Yoo YC, Matsuzawa K, Sato K, Saiki I, Tono-oka S, Samukawa K, Azuma I: Inhibitory effect of tumor metastasis in mice by saponins, ginsenoside-Rb2, 20(R)- and 20(S)- ginse-noside-Rg3, of red ginseng. Biol Pharm Bull 1995, 18:1197-1202.
43. Sato K, Mochizuki M, Saiki I, Yoo YC, Samukawa K, Azuma I: Inhibition of tumor angiogenesis and metastasis by a saponin of Panax ginseng, ginsenoside-Rb2. Biol Pharm Bull 1994, 17: 635-9.
44. Chen D, Zhao Y, Bai S, Shi Z, Zhang J. Effect of ginsenoside Rg3on the progression of orthotopically xenotransplant human breast cancer in nude mice and its mechanism. Sichuan Daxue Xuebao Yixue Ban 2003, 34: 546-8.
45. Kang XM, Zhang QY, Tong DD, Zhao W. Experimental study on anti-angiogenesis in mice with Lewis lung carcinoma by low dose of cyclophosphamide combined with ginsenoside Rg3. Zhongguo Zhongxiyi Jiehe Zazhi 2005, 25: 730-3.
46. Jerome Block, Steve Evans, INITIAL FORMAL CLINICAL STUDIES WITH THE GINSENG EXTRACT CARESENG®. ASCO Annual Meeting 2002.
47. X. Ouyang, Z. Yu, Z. Chen, F. Xie, W. Fang, Y. Peng, X. Chen, W. Chen, W. Wang, P. Qi, W. Jia. A Pilot Study of Safety and Efficacy of Pandimex with or without Paclitaxel in the Treatment of Advanced Solid Tumors. ASCO Annual Meeting, 2005, Abstract No: 3188.
48. Leung KW, Pon YL, Wong RN, Wong AS. Ginsenoside-Rg1 induces vascular endothelial growth factor expression through glucocorticoid receptor-related phosphatidylinositol 3-kinase/Akt and beta -catenin/TCF-dependent pathway in human endothelial cells. J Biol Chem 2006, 281:36280-88.
49. Bae EA, Shin JE, Kim DH. Metabolism of ginsenoside Red by human intestinal microflora and its estrogenic effect. Biol Pharm Bull 2005, 28:1903-08.
50. Cho J, Park W, Lee S, Ahn W, Lee Y. Ginsenoside-Rb1 from Panax ginseng C.A. Meyer activates estrogen receptor- alpha and -beta, independent of ligand binding. J Clin Endocrinol Metab 2004, 89:3510-15.
51. Furukawa T, Bai CX, Kaihara A, Ozaki E, Kawano T, Nakaya Y, Awais M, Sato M, Umezawa Y, Kurokawa J. Ginsenoside Re, a Main Phytosterol of Panax Ginseng, Activates Cardiac Potassium Channels via a Non- Genomic Pathway of Sex Hormones. Mol Pharmacol 2006, 70:1916-24.
Sherman Zhao, MD, PhD
Correspondence: Sherman_zhao@hotmail.com

