Yun Liu, Yufei Yang*, Yu Wu, Juan Liao
Department of Oncology, Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing 100091, China
ABSTRACT
OBJECTIVE To investigate the mechanisms of KangLaite Injection (KLT, a new anti-cancer drug) in the treatment of tumor by using CTL immune-related gene chip technique.
METHODS A total of 150 items of Oligo gene chip were selected, including 93 items of differential expression gene in our prophase experiments, 37 items of immuno-associated gene of tumor CTL cells searched from literatures and 20 items of house-keeping gene from Beijing Boao Bio-chip Company Ltd.
Forty T739 mouse weighting 18 to 22g (fifty male and fifty female) were allocated to forty groups randomly. Thirty of those were assigned to three groups signaled with KLT1, KLT2, KLT3 randomly, treated with KLT (25 mg/ kg body/day celiac injection) from the fourth day for 14 days, ten of those signaled with H treated with 0.9% Nacl (25mg body/day celiac injection) from the fourth day for 14 days as the negative control,, and ten mouse was normal, signaled with Z.
The mouse pulmonic tissues were mixed up orderly with the group for extracting mRNA separately. Each subarray of Oligo chips contained 450 genes (i.e., 3 same sites in the 150 gene). For each experiment, each gene was represented triplicate on each slide, and the experiments were performed in duplicate by dye swap, producing 6 data points, so that we determined the differential gene expression based on the statistical program t-test. Genes whose ratio lay outside the 95% confidence interval shown Statistically significant differences for expressed genes.
RESULTS AND DISCUSSION The difference of the same differential expression genes, filtered from 3 KLT samples chips was larger than 1.5-fold, and we got 10 items of tendency-identical genes, including 8 items of the gene being up-regulated expression, and 2 items exhibiting down-regulation. Comparing with normal control group, the tendency-identical genes had 6 items, among which the expression of tumor-inhibitory gene cyclin B2 in stage G2/M and blocking of the cellular G2/M-phase to reduce the abundant expression of cyclin B2 in stage G2/ M and blocking of the cellular G2/M to reduce the mitosis, the tumor cellular proliferation was thus suppressed. The accelerator of the mouse cyclin B2 is regulated by cell-cycle dependent cyclin (CDC), and the effect of CDC2 in promoting cell transformation is definitely associated with cyclin B2. Therefore, KLT induced up regulation of cyclin B2 expression may contribute to its effect in inhibiting the transformation of the tumor cells via checking CDC2 expression. Along with the development of tumor, the activity of alpha-1 protease inhibitor is suppressed. In the present study, after application of KLT, the alpha-1 expression was upregulated, shown that KLT may suppress the growth of tumor.
KEY WORDS Kanglaite injection; Gene expression; Cancer; Anti-cancer drug; Cellular proliferation
INTRODUCTION
Lung cancer is the most common cancer in both female and male persons all over the world, with estimated 1,352,132 new cases diagnosed and 1,178,918 deaths in 2002 (WHO 2002). The incidence is higher in developed and industrialized country than in developing country. Meta-analysis reveals that there was considerable pessimism about the role of chemotherapy in the treatment of non-small cell lung cancer [1]. There is no strong evidence that any regimen gives greater palliation. Higher dose regimens give more acute toxicity. There is evidence for a modest increase in survival (6% at 1 year and 3% at 2 years) in patients with better performance status given higher dose radiotherapy [2].
Kanglaite Injection (KLT) is a novel type of dysphasic anticancer injection prepared by extracting its efficacious anticancer component from a Chinese crude drug/coin seed for intravenous as well as intra-arterial injection. Currently, KLT has been the Basic Health Insurance Medicine of China. KLT has marked inhibitory effect on many tumors cell strains in in-vitro cultivation test such as mouse leukemic cells P388, L1210, human cervical carcinoma Hela S3, human colon Carcinoma M7609. It not only has the obviously anti-lung metastasis which has the similar inhibitory rate with Cyclophosphamide [3], but also can strengthens body’s immune functions. Experiences obtained from clinical application have demonstrated that KLT is significantly raise patient’s immune functions and improve his quality of life. Our previous investigation [4] find some differently expressed genes about immune such as IgE-BF, Titin, Lcp1 et al.
In this study, we have customed a mouse 55-mer oligonucleotide microarray to assess the gene expression profile of LA795 mouse with lung adenocarcinoma by Kanglaite Injection versus negative control, and normal lung also be investigated. We designed 150 mouse genes included 93 genes expressed significantly differently in our previous research, 37genes CTL cancer immunology genes and 20 house-keeping genes. Through the experiment, we went to research into the molecular mechanisms how Kanglaite Injection restrains cancer and to constitute potential novel therapeutic targets.
MATERIALS AND METHOD
RNA extraction
Forty T739 mouse weighting 18 to 22g (fifty male and fifty female) were randomized into forty groups. Thirty of those were randomized to three group signaled with KLT1, KLT2, KLT3, treated with KLT (25 mg/kg body/day celiac injection) from the fourth day for 14 days, ten of those signaled with H treated with 0.9% NaCl (25mg body/day celiac injection) from the fourth day for 14 days as the negative control, and ten mouse was normal, signaled with Z. Taken off the neck to kill them at the twentieth day, and taken the full lung out. The mouse lung tissues were mixed up orderly with the group, and snap-frozen in liquid nitrogen immediately after resection and then stored at -80 ℃.
RNA was extracted with TRIZOL reagent (Invitrogen, Gaithersburg, MD) and further purified
with a RNeasy mini kit (Qiagen, Valencia, CA) according to the manufacture’s instructions. The RNA quality was assessed by formaldehyde agarose gel electrophoresis and was quantitated spectrophotometrically.
Construction of Mouse Custom 55-mer oligonucleotide microarray
CTL immunology is the most important cancer immunology. Our previous microarrays research about KLT had filtered some genes related with cancer immunology such as M10062(Mouse IgG binding factor mRNA, IgE-BF), AA510391 (Ttid: Titin immunoglobulin domain protein), D37837 (Lcp1: lymphocyte cytosolic protein 1). We designed 150 mouse genes included 93 genes expressed significant difference in our previous research, 37genes CTL cancer immunology genes and 20 house-keeping genes.
An in-house high-throughput computer algorithm based on Linux operating system and Python programming language was employed to design 55-mer oligonucleotide probes for those 150 mouse genes. The fundamental rules of our computer algorithm include that all the probes should be close to 3’ end sequence, all oligonucleotides should have a close annealing temperature, and each oligonucleotide has less than or equal to 70% identity to all other mouse genes. The mouse custom oligonucleotide microarray was constructed in Capital biochip Corporation (Beijing, China). In a brief description, synthesized oligonucleotide was dissolved in 50% DMSO to a final concentration of 40 mM and printed on in-house manufactured amino silane glass slides. In addition, three Arabidopsis gene probes at a length of 55 bases were added as external controls. Arrays were spotted by using an OmniGrid ™ microarrayer (Genomic Instrumentation Services, Inc, San Carlos, CA). Each probe was spotted three times to facilitate subsequent data analysis. After printing, the slides were baked for 1h at 80℃ and stored dry at room temperature till use.
Prior to hybridization, the slides were rehydrated over 65℃ water for 10 seconds, snap dried on a 100℃-heating block for 5 seconds and UV cross-linked at 250 mJ/cm2. The immobilized oligonucleotides were washed off with 0.5% SDS for 15 min at RT and SDS was removed by dipping the slides in anhydrous ethanol for 30 seconds. The slides were spin-dried at 1000 rpm for 2 min.
Preparation of fluorescent dye-labeled DNA and hybridization
Fluorescent dye (cy5 and cy3-dCTP) labeled DNA was produced through Eberwine’s linear RNA amplification method and subsequent enzymatic reaction. In detail, double-stranded cDNA containing T7 RNA polymerase promoter sequence (5′-AAACG ACGGC CAGTG AATTG T AATA CGACT CACTA TAGGC GC-3’) was synthesized with 5 mg of total RNA using cDNA Synthesis System Kit according to manufacturer recommended protocol (TaKaRa, Dalian, China). A T7-OligodT primer (5’- AAACG ACGGC CAGTG AATTG TAATA CGACT CACTA TAGGC GC TT TTT TTT TTT TTT TTTV –3’) was in replacement of poly T primer provided in the kit.
After completion of ds cDNA synthesis, ds cDNA was purified with PCR Purification Kit (Qiagen), and the final cDNA were eluted in 60 ml Elution Buffer. A half of the eluted ds-strand cDNA product was vacuumed to 8 ml and subject to in vitro transcription reaction in 20 ml of reaction system using T7 RiboMAX™ Express Large Scale RNA Production System (Promega, Madison, WI). Reaction occurred for 3 h at 37℃ and amplified RNA (aRNA) was purified with RNeasy Mini kit (Qiagen). Considering of the more convenient manipulation with DNA than with RNA, some researchers are apt to label DNA in subsequent reverse transcription reaction instead of labeling RNA directly in vitro transcription [5]. Because sense oligonucleotide arrays were used here, we took a cDNA labeling approach with Klenow enzyme after reverse transcription. The labeling strategy using klenow enzyme has been introduced into probe labeling in microarray technology by other researchers [6]. We found the Klenow enzyme possesses high labeling efficiencies and the labeled DNAs have shorter length (100-400 bp), facilitating the hybridization procedure. Briefly, 1 mg aRNA was mixed with 2 mg of random hexamer, denatured at 70℃ for 5 min and cooled on ice. Then 4 ml of first strand buffer, 2 ml of 0.1M DTT, 1ml 10 mM dNTP, and 1.5 ml Superscript II (Invitrogen) were added. Tubes were incubated at 25℃ for 10 min then 42℃ for 60 min. The products were purified using a PCR purification kit (Qiagen) and vacuumed to 10 ml. cDNA was mixed with 2 mg random nonamers, heated to 95° C for 3 min and snap cooled on ice. 10×buffer, dNTP and Cy5-dCTP or Cy3-dCTP (Amersham Pharmacia Biotech, Piscataway, NJ) were added at final concentration of 120 mM each dATP, dGTP, dTTP, and 60 mM dCTP and 40 mM Cy-dye respectively. Klenow enzyme (1 ml, Takara) was added, and reaction was performed at 37℃for 60 min. The labeled DNA was purified with a PCR purification kit (Qiagen), resuspended in Elution buffer and check O.D. Labeled control and test samples were quantitatively adjusted based on the efficiency of Cy-dye incorporation and mixed into 12 mL hybridization solution (3×SSC, 0.2% SDS, 25% formamide and 5×Denhardt’s). DNA in hybridization solution was denatured at 95℃ for 3 min prior loading on a microarray. The array was hybridized at 42℃ overnight and washed with two consecutive washing solutions (0.2% SDS, 2×SSC at 42°C for 5 min, and 0.2% SSC for 5 min at room temperature).
Imaging and data analysis
Arrays were scanned with a ScanArray Express scanner (Packard Bioscience, Kanata, OT), and obtained images were analyzed with GenePix Pro 4.0 (Axon Instruments, Foster City, CA). A space and intensity-dependent normalization based on a LOWESS program [7] was employed here. For each experiment, each gene was represented triplicate on each slide, and the experiments were performed in duplicate by dye swap, producing 6 data points, so that we determined the differently expressed genes based on the statistical program t-test. Genes whose ratio lay outside the 95% confidence interval were determined to be significantly differently expressed genes.
RESULTS
Verification of micro array technique system
There were 450 spots on each slide, each gene repeated three times. The genes including 93 genes expressed significant difference in our previous research about KLT, 37 genes CTL cancer immunology genes, 20 housekeeping genes as positive control and 4 spotting solution which served as negative control. In present study, the individual result of eight hybridization showed that all positive control signals are distinct, and all of negative control signals were very low. These proved the reliability of the data.
Judgement of differentially expressed genes
The standard of determination for differentially expressed genes was that the absolute value of natural logarithm of the mean ratio was greater than 1.5 or smaller than 0.67, that was to say change of gene expression was above 1.5 times, and the signal value of either Cy3 or Cy5 needed to be greater than 800.
Differential Gene expression
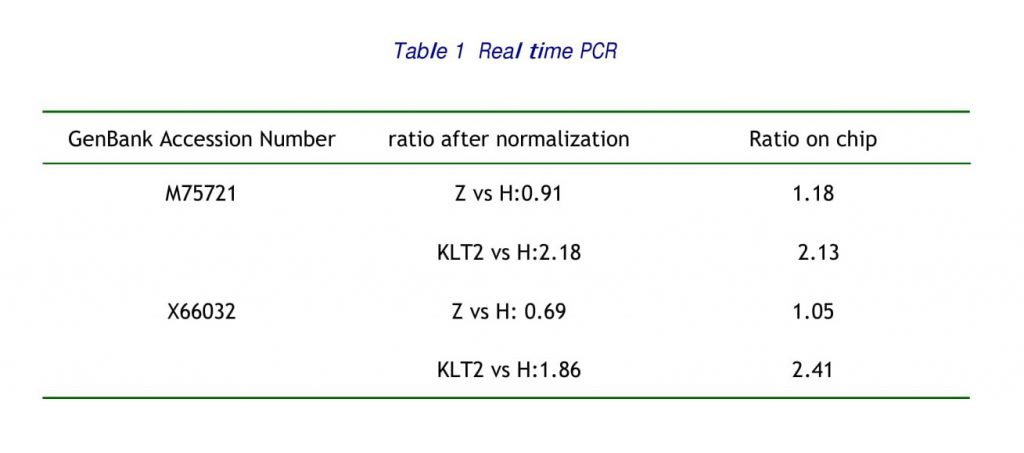
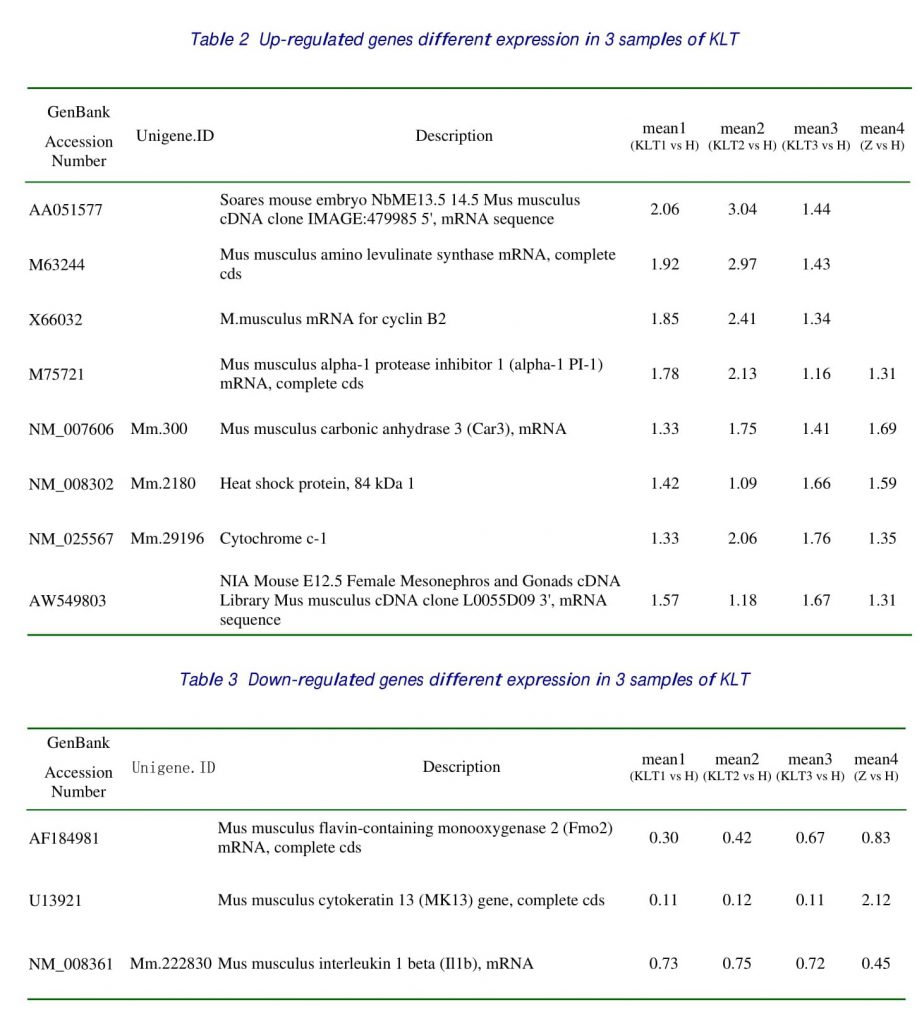
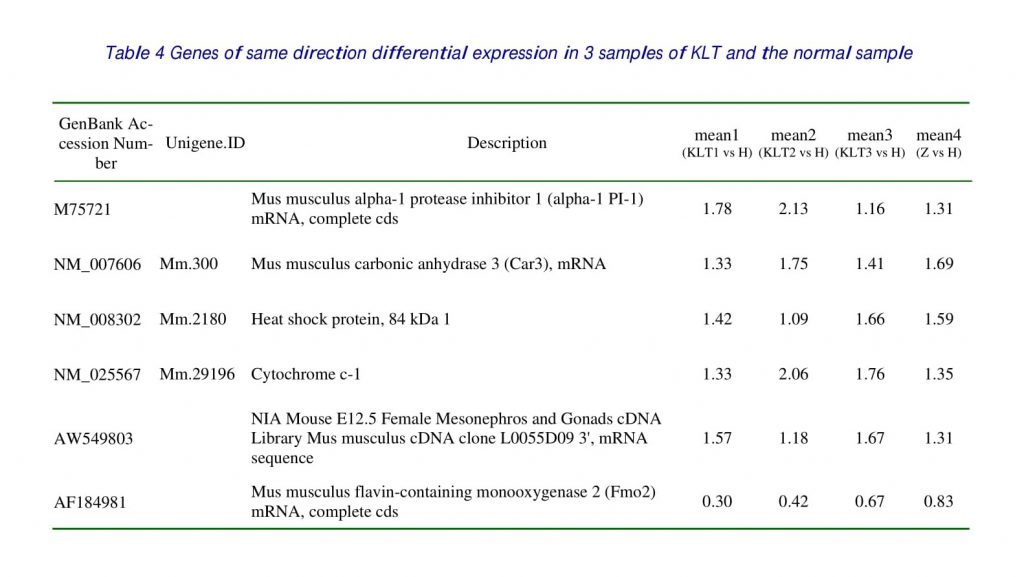
Among 4 samples investigated, 10 genes, which accounted for 6.67 % of genes on the microarray slides, exhibited differential expression at least in 2 and had the same direction of expression in 3 samples about KLT. There were 8 over-expressed genes including 4 genes that had the same direction of differential expression with the normal sample. There were 2 under-expression genes including one gene that had the same direction of differential expression with the normal sample and one gene had the differential expression. The genes, which took superior places of differential expression, were listed in Table 1, 2, 3, 4.
DISCUSSION
M75720, alpha-1 protease inhibitor 1 (alpha-1 PI-1)
Alpha-1 proteinase inhibitor (alpha-1 PI) is the main serine proteinase inhibitor found in human plasma and is a potent elastase inhibitor in various tissues, including lung. Lung Inhibition of a cell surface proteinase can inhibit the growth of many normal human cell types in culture. Some tumours cells are also sensitive to proteinase inhibitors, but others are resistant, and continue to grow in the presence of these inhibitors [8]. Proteolytic enzymes could be very important to spread of cancer. One such inhibitor is the serum glycoprotein, alpha-1 proteinase inhibitor. By means of high-performance anion-exchange chromatography, cancer API was shown to contain more fucose and less N-Acetylglucosamine than healthy API.
A competitive cDNA library screening (CCLS) was employed to determine that alpha-1 protease inhibitor was overexpressed in chemically induced pulmonic adenomas compared with paired normal pulmonic tissues [9]. Several studies have demonstrated the hepatocarcinogenicity of Dichloroacetic acid (DCA) in mice when administered in drinking water. The expression of alpha-1 protease inhibitor was suppressed in the DCA-treated mice [10]. Murine tumor development (hepatoma HA-1, lymphosarcoma LS, Lewis pulmonic adenocarcinoma) was followed by a decreased activity of serum alpha-1-proteinase inhibitor both in successfully treated and untreated groups. According to data of literature, similar dated were obtained in humans with tumors. It was suggested that changes of expression of alpha-1-proteinase inhibitor by tumors and its secretion were involved in decreased activity of alpha-1-proteinase inhibitor [11]. The proportion of serum PSA-alpha 1-protease inhibitor of total PSA was lower in cancer cases than in controls (0.9% versus 1.6%, p<0.001) [12].
Alpha-1-proteinase inhibitor belongs to acute phase reactant in human but not in mice [11]; Dexamethasone, the glucocorticoid analog significantly reduced the basal level of alpha-1-PI expression as well as antagonized the effect of lipopolysaccharide and interleukin-6, stimulators of alpha 1-PI synthesis in cells of monocyte/macrophage lineage. Since increased levels of all these mediators are observed in both acute infections and inflammatory reactions, their combination may affect the contribution of monocytes to the synthesis of alpha-1-PI [13]. Levels of BALF alpha -1-PI were significantly increased in patients with lung cancer compared with benign pulmonary diseases. The local increase of alpha-1-PI in lung cancer may act as a humoral inhibitor against the production of IL-1 and TNF by alveolar macrophages [14].
Mouse T739 inoculating with tumor LA795 (mouse pulmonic adenocarcinoma) is not only the tumor metastasis model that killed at the 20th day, but also the cancer cachectic model that killed at the 22nd day [15,16]. Cancer cachectic is the main reason leading to death. The occurring mechanisms of it is unclear and how to treat it is unknown. Current research proved that the occurring of cancer cachectic was regulated by TNF-α, IL-1, IL-6, IL-10, and IL-12[17, 18, 19, 20, 21, 22, 23]. The laboratory results of cytokines associated with cachexia, have revealed that the Serum TNF-αand IL-1 levels were decreased by KLT(P<0.05) [24].
In this study, in contrast with control samples inoculated with pulmonic adenocarcinoma, the expression of alpha-1 PI-1 was upregulated as 1.78, 2.13 and 1.16 in the treatment with KLT that the growth of tumor cell was restrained (table3) and had not differential expression in the normal samples. And have been proved by the real time PCR. The expression of IL-1 was down-regulation in three samples of KLT similarly as 0.73, 0.75, 0.72, but the expression of normal sample was 0.45. KLT inhibit pulmonic adenocarcinoma cells metastasis through up-regulated alpha-1 PI-1 that act as a humoral inhibitor against the production of IL-1 by alveolar macrophages.
X66032 CCNB2 cyclin B2
Cyclin B2 is one of the tumor suppressor gene that is a key event in many cancers [25]. Cyclin B is an important regulator of progression through the cell division cycle. In many vertebrates, cyclin B has several subtypes, but the functional differences among them are largely unclear. Cyclins are essential regulators of the cell division cycle. Cyclin B associates with the cyclin-dependent kinase 1 (cdc2) to form a complex which is required for cells to undergo mitosis. In mammalian cells three B-type cyclins have been characterised, cyclin B1, B2 and B3. The oscillating appearance of cyclin B1 and B2 proteins during the cell cycle is in part due to fluctuating mRNA levels. The cell cycle-dependent synthesis of cyclin B1 and B2 has been investigated in detail displaying maximum expression in G2 which is mainly regulated on the transcriptional level. This regulation of the mouse cyclin B2 promoter is controlled by a tandem promoter element named cell cycle-dependent element (CDE) and cell cycle genes homology region (CHR) which regulates cell cycle-dependent transcription of cdc25C, cyclin A and cdc2[26]. Cyclin B2 transcription is repressed through a novel CDE/CHR element in resting and G1 cells. By relief of this repression in S and G (2) oscillating expression of cyclin B2 mRNA is achieved during the cell cycle [27].
Overexpression of cyclins B, resulting from different mechanisms, could contribute, through an alteration of the spindle checkpoint, to the chromosomal instability observed in cancer [28]. Xenopus cyclin B2 is involved in bipolar spindle formation through its cytoplasmic retention signal (CRS) region. The CRS region, especially its C-terminal seven acidic residues, of cyclin B2 is required for bipolar spindle formation in both the meiotic and mitotic cell divisions [29]. Cdc2 increases cell migration via specific association with cyclin B2, cdc2 is a downstream effector of the alphavbeta3 integrin, and that it promotes cell migration [30]. Mitotic progression is timely regulated by the accumulation and degradation of A- and B-type cyclins. The induction of cyclin B2 expression in cultured cells during G2 phase accelerates the entry into mitosis and allows cells to override the replication checkpoint induced by hydroxyurea in the simultaneous presence of caffeine or okadaic acid, drugs that are known to alleviate checkpoint control [31]. DNA microarray analysis of 99 lung tumor samples and 15 normal lung tissues revealed that receptor for advanced glycation end products (RAGE) mRNA is reduced fourfold (p=7.8×10(-11)) and cyclin-B2 mRNA is upregulated twofold (p=5.9×10(-18)) in lung carcinoma compared with normal lung. In 94.7% of the samples this quotient correctly distinguished non-small cell lung cancer from normal pulmonic tissue, suggesting the RAGE/cyclin-B2 quotient as a potential means for diagnosis of lung cancer [32]. Upon treatment of HepG2 cells with 5-fluorouracil or methotrexate, p53 levels increase while concentrations of cyclin B2 mRNA, measured by RT-PCR with the Light Cycler system, are reduced. One way of regulating G2 arrest may be a reduction in cyclin B levels through p53-dependent transcriptional repression [33]. A sustained increase in cyclin B2 concentrations in the cells shown by immunoblotting indicate that JV-1-38 causes a block at the end of the G2 phase of cell cycle [34].
KLT has a marked effect on the cell progression of K562 cells with a dosage dependency. When treated with a dosage of 1 ul/ml for 48hr, the percentage of cells at S and G2+M phase increased markedly, while cells at G1 Phase decreased evidently; When the dosage was increased to 5 ul/ml, the percentage of cells at S phase decreased apparently, while cells at G2+M phase remarkably rose; when the dosage was increased to 10 ul/ml, Cells at S phase declined to only 11.6% of the control group, while cells at G2+M increased by 11 times that of the control group. When the dosage was raised to as high as 50ul/ml, all the cells underwent death totally. At this time, it was not possible to analyze the distribution of the cells in the cell cycle. The above results indicated that, the key link of KLT’s actions was mainly the inhibition of the cells at G2+M phase, thus reduced the number of cells entering G0 and G1 phase and leading to the reduction in percentage of cells at S phase and decreased the mitosis of tumor cells, and then their multiplication.
The expression of cyclin B2 was up regulated in the 3 samples of KLT and had not differential expression in the normal samples. The expression of tumor-inhibitory gene cyclin B2 in stage G2/M and blocking of the cellular G2/M-phase to reduce the abundant expression of cyclin B2 in stage G2/M and blocking of the cellular G2/M to reduce the mitosis, the tumor cellular proliferation was thus suppressed. The accelerator of the mouse Cyclin B2 is regulated by cell-cycle dependent cyclin (CDC), and the effect of CDC2 in promoting cell transformation is definitely associated with Cyclin B2. Therefore, KLT induced upregulation of Cyclin B2 expression may contribute to its effect in inhibiting the transformation of the tumor cells via checking CDC2 expression. That is probably the key link of the mechanism of KLT’s antitumor actions.
NM_025567 Mm.29196 Cytochrome c-1
When Prof. Taniai M et al. Investigation Mcl-1 mediates tumor necrosis factor-related apoptosis inducing ligand resistance in human cholangiocarcinoma cells, they demonstrated that TRAIL-mediated apoptosis in the stably transfected cells was associated with mitochondrial depolarization, Bax activation, cytochrome c release from mitochondria, and caspase activation [35,36]. Tumors induce T-cell apoptosis as a mechanism of inhibiting antitumor immunity. Using coculture experiments, it has been shown that tumor lines stimulate T-cell apoptosis by a pathway involving a mitochondrial permeability transition and cytochrome c release [37].
The expression of Cytochrome c-1 was upregulated in the three samples of KLT as 1.33, 2.06, and1.76, it was 1.35 in the normal samples. It was means that tumor lines stimulate T-cell apoptosis by a pathway involving cytochrome c release. KLT could induce cytochrome c release to inhibit T-cell apoptosis that would improve antitumor immunity.
NM_007606 Mm.300 Mus musculus carbonic anhydrase 3 (Car3)
Carbonic anhydrases (CAs, EC 4.2.1.1) are widespread enzymes, present in mammals in at least 14 different isoforms: some of these isozymes are cytosolic (CA I, CA II, CA III, CA VII), while others are membrane-bound (CA IV, CA IX, CA XII and CA XIV); CA V is mitochondrial, and CA VI is secreted in the saliva. Three catalytic forms are also known (CARP VIII, CARP X and CARP XI). many potent CAIs were shown to inhibit the growth of several tumor cell lines in vitro and in vivo [38]. Carbonic anhydrases (CA) influence intra-and extracellular pH and ion transport in varied biological processes [39]. Prof. Lin L et al. detected that carbonic anhydrase III was under-expression or absent in mouse pulmonic adenocarcinoma compared with normal lungs [40].
This study also proved that carbonic anhydrase III was under expression or absent in mouse pulmonic adenocarcinomas compared with normal lungs. The expression of carbonic anhydrase III was upregulated in the samples treated with KLT as 1.33, 1.75, and 1.41. It was 1.69 in the normal samples. That would illustrate KLT could get back the expression of carbonic anhydrase III to the normal level.
AF184981 Mus musculus flavin-containing monooxygenase 2 (Fmo2) mRNA
Mammalian flavin-containing monooxygenase (FMO) exists as six gene families and metabolizes a plethora of drugs and xenobiotics. A more recent ethnically related polymorphism in expression of the major FMO in lung, FMO2, has been described. All Caucasians and Asians genotyped to date are homozygous for a CAG –> TAG amber mutation resulting in a premature stop codon and a non-functional protein truncated at AA 472 (wildtype FMO2 is 535 AA). Preliminary evidence indicates that FMO2.1 is very active toward the S-oxygenation of low MW thioureas, including the lung toxicant ethylene thiourea. Polymorphic expression of functional FMO2 in the individuals of African and Hispanic descent may markedly influence drug metabolism and/or xenobiotic toxicity in the lung [41]. The major FMO in lung of most mammals, including non-human primates, is FMO2[42]. Generally, the flavin-containing monooxygenase of mammalian systems has been considered a detoxication enzyme converting nucleophilic heteroatom-containing chemicals into polar, more readily excreted metabolites. The Arabidopsis FMOlike gene product, while resembling the mammalian counterpart in many respects, is quite distinct. However, study of the Arabidopsis FMO-like enzyme may provide considerable insight into the possible role of mammalian FMOs in biogenic amine metabolism [43]. FMOs may also play a role in the in vivo metabolism of endogenous homocysteine S-conjugates [44].
In this study, the expression of FMO2 was downregulated in the samples treated with KLT as 0.30, 0.42, and 0.67, it was 0.83 in the normal samples. It was proved that KLT may down-regulated FMO2 to regulate endogenous homocysteine S-conjugates in the in vivo metabolism.
NM_008302, Mm.2180, Heat shock protein, 84 kDa 1
Heat shock protein-based vaccines have been shown to immunize against cancer and infectious diseases in both prophylactic and therapeutic protocols [45]. At the permissive temperature, hsp84 resided in the cytoplasm and little or no hsp84 formed a complex with p53. The results suggest that hsp84 binds mutant p53 in a spatial and/or conformation dependent manner [46].
M63244, Mus musculus amino levulinate synthase mRNA
Iron might play its role also at the pre-translational level of the expression of ALAS-E or in the stability of (ALAS-E) mRNA [47].
The key rate-controlling enzyme of the heme biosynthetic pathway is 5-aminolevulinate synthase (ALAS) and an erythroid-specific isoform (ALAS2) is up-regulated during erythropoiesis. Differentiation of embryonic stem cells with a disrupted ALAS2 gene has established that expression of this gene is critical for erythropoiesis and cannot be compensated by expression of the ubiquitous isoform of the enzyme (ALAS1) [48].
The expression of amino levulinate synthase was up regulated in the sample’s treatment with KLT as 1.92, 2.97, and1.43, there was no different expression in the normal samples. KLT may up regulated the expression of amino levulinate synthase. That is probably the key link of the mechanism of KLT’s antitumor actions.
REFERENCES
1 Non-small Cell Lung Cancer Collaborative Group. Chemotherapy for non-small cell lung cancer (Cochrane Review). In: The Cochrane Library, Issue 3, 2004. Chichester, UK: John Wiley & Sons, Ltd.
2 Macbeth F, Toy E, Coles B, Melville A, Eastwood A. Palliative radiotherapy regimens for non-small cell lung cancer (Cochrane Review). In: The Cochrane Library, Issue 3, 2004. Chichester, UK: John Wiley & Sons, Ltd.
3 Aiguo Liu, Tongdu Li, Yisheng Tao, Zhenshan Xu, Qi Niu, Huiming Dong, Desheng Song. Collection of the Studies of Kanglaite Injection Against Tumors [M] Zhejiang University Press, Zhejiang, 1998, 107-109.
4 Wu Yu, Yang Yufei, Wu Donghua. Study on the expression pattern of KANGLAITE of Anti-lung metastasis of LA795 mouse. Chinese Journal of Lung cancer 2003, 6(6): 473-476.
5 Gomes LI, Silva RL, Stolf BS, Cristo EB, Hirata R, Soares FA, Reis LF, Neves EJ, Carvalho AF. Comparative analysis of amplified and nonamplified RNA for hybridization in cDNA microarray. Anal Biochem 2003; 321(2): 244-251.
6 Gomes LI, Silva RL, Stolf BS, Cristo EB, Hirata R, Soares FA, Reis LF, Neves EJ, Carvalho AF. Comparative analysis of amplified and nonamplified RNA for hybridization in cDNA microarray. Anal Biochem 2003; 321(2): 244-251.
7Yang YH, Dudoit S, Luu P, Lin DM, Peng V, Ngai J, Speed TP. Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res 2002; 30(4): e15.
8 Scott GK, Tse CA. Changes in sensitivity of human tumour cells to growth inhibition by proteinase inhibitors. Cell Biol Int. 1994 Feb; 18(2): 89-93.
9 Yao R, Wang Y, Lubet RA, You M. Differential gene expression in chemically induced mouse lung adenomas. Neoplasia. 2003 Jan-Feb; 5(1):41-52.
10 Thai SF, Allen JW, DeAngelo AB, George MH, Fuscoe JC. Detection of early gene expression changes by differential display in the livers of mice exposed to dichloroacetic acid. Carcinogenesis. 2001 Aug; 22(8):1317-22.
11 Korolenko TA, Levina OA, Falameeva OV, Tolochko ZS, Spiridonov VK, Andreeva EM, Il’nitskaia SI, Kaledin VI. Comparative characteristic of serpin–alpha-1 proteinase inhibitor in human and mice serum. Ross Fiziol Zh Im I M Sechenova. 2003 Apr; 89 (4): 420-6.
12 Finne P, Zhang WM, Auvinen A, Leinonen J, Maattanen L, Rannikko S, Tammela TL, Stenman UH. Use of the complex between prostate specific antigen and alpha 1-protease inhibitor for screening prostate cancer. J Urol. 2000 Dec; 164(6):1956-60.
13 Cichy J, Potempa J, Travis J. Effect of dexamethasone on Kanglaite Injection alpha 1-proteinase inhibitor synthesis in human cells of monocytic origin. Biochem Biophys Res Commun. 1995 Mar 8; 208(1): 216-22.
14 Ameshima S, Ishizaki T, Takahashi H, Kishi Y, Sasaki F, Nakai T, Miyabo S. Inhibitory effects of alpha 1 protease inhibitor on the production of IL-1 and TNF alpha by alveolar macrophages in patients with lung cancer. Nihon Kyobu Shikkan Gakkai Zasshi. 1992 May; 30(5): 873-80.
15 liu Aiguo, Li Tongdu, Tao yisheng, Xu Zhenshan, Niu qi, Dong huiming, Song Desheng. Collection of the Studies of Kanglaite Injection Against Tumors [M], Zhejiang University Press, Zhejiang 1998, 115-118.
16 Li Tongdu et al. Experimental Study of the Counteractive Effect of Kanglaite Injection on Cancerous Cachexia, Chinese Journal of Clinical Oncology 1998, 25(2): 131.
17 Steiner H, Godoy-Tundidor S, Rogatsch H, et al. Accelerated in vivo growth of prostate tumors that up-regulate interleukin-6 is associated with reduced retinoblastoma protein expression and activation of the mitogen-activated protein kinase pathway. Am J Pathol. 2003 Feb; 162(2): 655 – 63.
18 Carbo N, Busquets S, van Royen M, TNF-alpha is involved in activating DNA fragmentation in skeletal muscle. Br J Cancer. 2002 Mar 18; 86(6): 1012-6.
19 Buck M, Zhang L, Halasz NA, et al. nuclear export of phosphorylated C/EBPbeta mediates the inhibition of albumin expression by TNF-alpha. EMBO J. 2001 Dec 3; 20(23): 6712-23.
20 Tessitore L, Vizio B, Jenkins O, et al. Leptin expression in colorectal and breast cancer patients. Int J Mol Med. 2000 Apr; 5(4): 421-6.
21 Hou L, Sasaki H, Stashenko P. Toll-like receptor 4-deficient mice have reduced bone destruction following mixed anaerobic infection. Infect Immun. 2000 Aug; 68(8): 4681-7.
22 Cahlin C, Korner A, Axelsson H, et al. Experimental cancer cachexia: the role of host-derived cytokines interleukin (IL)-6, IL-12, interferon-gamma, and tumor necrosis factor alpha evaluated in gene knockout, tumor-bearing mice on C57 Bl background and eicosanoid-dependent cachexia. Cancer Res.2000 Oct 1; 60(19): 5488-93.
23 Barber MD, Powell JJ, Lynch SF, et al. A polymorphism of the interleukin-1 beta gene influences survival in pancreatic cancer. Br J Cancer. 2000 Dec; 83(11): 1443-7.
24 Li Tongdu et al. Experimental Study of the Counteractive Effect of Kanglaite Injection on Cancerous Cachexia, Chinese Journal of Clinical Oncology 1998, 25(2): 131.
25 Lee HS, Lee HK, Kim HS, Yang HK, Kim WH. Tumour suppressor gene expression correlates with gastric cancer prognosis. J Pathol. 2003 May; 200(1): 39-46.
26 Wasner M, Haugwitz U, Reinhard W, Tschop K, Spiesbach K, Lorenz J, Mossner J, Engeland K. Three CCAAT-boxes and a single cell cycle genes homology region (CHR) are the major regulating sites for transcription from the human cyclin B2 promoter. Gene. 2003 Jul 17; 312: 225-37.
27 Lange-zu Dohna C, Brandeis M, Berr F, Mossner J, Engeland K. A CDE/CHR tandem element regulates cell cycle dependent repression of cyclin B2 transcription. FEBS Lett. 2000 Nov 3; 484(2): 77-81.
28 Sarafan-Vasseur N, Lamy A, Bourguignon J, Pessot FL, Hieter P, Sesboue R, Bastard C, Frebourg T, Flaman JM. Overexpression of B-type cyclins alters chromosomal segregation. Oncogene. 2002 Mar 27; 21(13): 2051-7.
29 Yoshitome S, Furuno N, Hashimoto E, Sagata N. The Cterminal seven amino acids in the cytoplasmic retention signal region of cyclin B2 are required for normal bipolar spindle formation in Xenopus oocytes and embryos. Mol Cancer Res. 2003 Jun; 1(8): 589-97.
30 Manes T, Zheng DQ, Tognin S, Woodard AS, Marchisio PC, Languino LR. Alpha(v)beta3 integrin expression upregulates cdc2, which modulates cell migration. J Cell Biol. 2003 May 26; 161(4): 817-26.
31 Weingartner M, Pelayo HR, Binarova P, Zwerger K, Melikant B, de la Torre C, Heberle-Bors E, Bogre L. A plant cyclin B2 is degraded early in mitosis and its ectopic expression shortens G2-phase and alleviates the DNA-damage checkpoint. J Cell Sci. 2003 Feb 1; 116(Pt 3): 487-98.
32 Hofmann HS, Hansen G, Burdach S, Bartling B, Silber RE, Simm A. Discrimination of human lung neoplasm from normal lung by two target genes. Am J Respir Crit Care Med. 2004 Sep 1; 170(5): 516-9.
33 Krause K, Wasner M, Reinhard W, Haugwitz U, Dohna CL, Mossner J, Engeland K. The tumour suppressor protein p53 can repress transcription of cyclin B. Nucleic Acids Res. 2000 Nov 15; 28(22): 4410-8.
34 Szepeshazi K, Schally AV, Armatis P, Groot K, Hebert F, Feil A, Varga JL, Halmos G. Antagonists of GHRH decrease production of GH and IGF-I in MXT mouse mammary cancers and inhibit tumor growth. Endocrinology. 2001 Oct; 142(10): 4371-8.
35 Taniai M, Grambihler A, Higuchi H, Werneburg N, Bronk SF, Farrugia DJ, Kaufmann SH, Gores GJ. Mcl-1 mediates tumor necrosis factor-related apoptosis-inducing ligand resistance in human cholangiocarcinoma cells. Cancer Res. 2004 May 15; 64(10): 3517-24.
36 Henson ES, Gibson EM, Villanueva J, Bristow NA, Haney N, Gibson SB. Increased expression of Mcl-1 is responsible for the blockage of TRAIL-induced apoptosis mediated by EGF/ErbB1 signaling pathway. J Cell Biochem. 2003 Aug 15; 89(6): 1177-92.
37 Molto L, Rayman P, Paszkiewicz-Kozik E, Thornton M, Reese L, Thomas JC, Das T, Kudo D, Bukowski R, Finke J, Tannenbaum C. The Bcl-2 transgene protects T cells from renal cell carcinoma-mediated apoptosis. Clin Cancer Res.2003 Sep 15; 9(11): 4060-8.
38 Supuran CT, Vullo D, Manole G, Casini A, Scozzafava A. Designing of Novel Carbonic Anhydrase Inhibitors and Activators. Curr Med Chem Cardiovasc Hematol Agents. 2004 Jan; 2(1): 51-70.
39 Wykoff CC, Beasley N, Watson PH, Campo L, Chia SK, English R, Pastorek J, Sly WS, Ratcliffe P, Harris AL. Expression of the hypoxia-inducible and tumor-associated carbonic anhydrases in ductal carcinoma in situ of the breast. Am J Pathol. 2001 Mar; 158(3): 1011-9.
40 Lin L, Wang Y, Bergman G, Kelloff GJ, Lubet RA, You M. Detection of differentially expressed genes in mouse lung adenocarcinomas. Exp Lung Res. 2001 Apr-May; 27(3): 217-29.
41 Krueger SK, Williams DE, Yueh MF, Martin SR, Hines RN, Raucy JL, Dolphin CT, Shephard EA, Phillips IR. Genetic polymorphisms of flavin-containing monooxygenase (FMO). Drug Metab Rev. 2002 Aug; 34(3): 523-32.
42 Henderson MC, Krueger SK, Siddens LK, Stevens JF, Williams DE. S-oxygenation of the thioether organophosphate insecticides phorate and disulfate by human lung Flavin containing monooxygenase 2. Biochem Pharmacol. 2004 Sep 1; 68(5): 959-67.
43 Cashman JR. Human and plant flavin-containing monooxygenase N-oxygenation of amines: detoxication vs. bioactivation. Drug Metab Rev. 2002 Aug; 34(3): 513-21.
44 Elfarra AA. Potential role of the flavin-containing monooxygenases in the metabolism of endogenous compounds. Chem Biol Interact. 1995 Apr 28; 96(1): 47-55.
45 Menoret A, Bell G. Purification of multiple heat shock proteins from a single tumor sample. J Immunol Methods. 2000 Apr 3; 237(1-2): 119-30.
46 Sepehrnia B, Paz IB, Dasgupta G, Momand J. Heat shock protein 84 forms a complex with mutant p53 protein predominantly within a cytoplasmic compartment of the cell. J Biol Chem. 1996 Jun 21; 271(25): 15084-90.
47 Fuchs O, Ponka P. The role of iron supply in the regulation of 5-aminolevulinate synthase mRNA levels in murine erythroleukemia cells. Neoplasma. 1996; 43(1): 31-6.
48 Sadlon TJ, Dell’Oso T, Surinya KH, May BK. Regulation of erythroid 5-aminolevulinate synthase expression during erythropoiesis. Int J Biochem Cell Biol. 1999 Oct; 31(10):1153-67.
*Correspondence to:
Yufei Yang, MD, PhD
Department of Oncology, Xiyuan Hospital of Traditional Chinese Medicine of China, Beijing 100091, China
Telephone: +86-01-62875599-6159
Fax: 62872221
Email: yyf93@vip.sina.com

