Ying Gao, Jun Chen, Mingyan Wang,
Jinhua Xu, Dong Wang, Baochang Cai
Nanjing University of Traditional Chinese Medicine, Nanjing 210029, China
Jiangsu Key Laboratory of Chinese Medicine Processing, Nanjing 210029, China
ABSTRACT
Objective:
To select a tumor cell line that was the most sensitive to brucine.
Methods:
Thiazolyl blue (MTT) test was used to examine the proliferation inhibition effects of brucine on human hepatoma cell line (SMMC-7721), human pulmonary cancer cell line (A549), human cervical cancer cell line (HeLa), human breast carcinoma cell line (MDA-MB-231), human colon carcinoma cell line (LoVo), human stomach poorly differentiated adenocarcinoma cell line (BGC-823), human gastric cancer cell line (SGC-7901, MKN-45), and human ovarian carcinoma cells (A2780). The optimal function time of brucine on all kinds of cell lines were also determined. The effect of brucine on apoptosis of A2780 was investigated by using Hoechst 33258 staining, double fluorescence staining of acridine orange (AO) and ethidium bromide (EB), flow cytometry with PI staining and Annexin V and PI double staining.
Results:
Among 9 cell lines, A2780 cell line was the most sensitive to brucine, and the 50% inhibition concentration (IC50) of brucine for A2780 cells was 1.43μM. Partial cells presented the characteristic morphological changes of apoptosis under fluorescence microscope. Brucine caused most of A2780 cells arrested in S phase while minor arrested in G2/M phase.
Conclusion:
Brucine had significant inhibition effect on A2780 in a dose-dependent and time-dependent manner (P<0.05). The results of flow cytometry displayed the mechanism might be related to the cell cycle arrested and the cell apoptosis induced by brucine.
Key Words:
Brucine; Antitumor; Apoptosis
INTRODUCTION
Nux vomica, the dried seed of Strychnos nux-vomica L. (Loganiaceae) has been effectively used in Chinese folk medicine to treat tumor-related diseases. For example, it is a major ingredient in some herbal prescriptions such as “Ping-xiao” capsule, “Ci-dan” capsule, “Ma-Qian-zi” tablet to reduce the occurrence of malignancies such as liver cancer [1]. In addition, nux vomica is also claimed to improve blood circulation or relieve rheumatic pain [2]. Alkaloids are the main ingredients in nux vomica and mainly responsible for the pharmacological properties possessed by nux vomica. In our previous study, 16 alkaloids have been isolated and identified from the crude nux vomica, 80% of which are strychnine and brucine, as well as their derivatives such as brucine N-oxide or isostrychnine [3]. Some alkaloids from nux vomica were shown to inhibit the cell growth of HeLa and K562[4] and in our previous study, we found that in contrast to brucine (Fig.1), brucine N-oxide did not show any cytotoxic effect on HepG2 cells; meanwhile, neither strychnine nor isostrychnine exhibited a stronger effect than that of brucine [5].
The aim of the present study was to identify which type of the brucine is mainly responsible for the anti-tumor effect exerted by nux vomica as well as its underlying mechanisms. To achieve this goal, nine tumor cell lines were applied to test the cell survival rate and cytotoxicity with 3-(4,5-dimethyl-2-thiazolyl)-2, 5-diphenyl tetrazolium bromide (MTT) assay. The results of this investigation might provide evidence to determine whether brucine could be used as a promising anticancer chemotherapeutic drug.
MATERIALS AND METHODS
Materials
Brucine was supplied by Carl Roth GmbH + Co. (German), Hoechst Staining Kit was purchased from Beyotime Institute of Biotechnology (Nantong, China). Propidium iodide (PI), acridine orange (AO), ethidium bromide (EB), Annexin V-FITC Apoptosis Detection Kit were purchased from Nanjing KeyGen Biotech Co. Ltd (Nanjing, China). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrasolium bromide (MTT) was purchased from Sigma. Brucine was dissolved in RPMI 1640 medium (Gibco, USA). Adjustment of the solutions to pH 7.2–7.4 was made with HCl (0.5 M) or NaOH (0.5 M) if necessary. All solutions were filtered through a 0.22 μm filter membrane (GVMP 01230, Millipore) and stored at 4°C until use.
Cell culture
Human hepatoma cell line (SMMC-7721), human cervical cancer cell line (HeLa) were purchased from Nanjing KeyGen Biotech Co. Ltd (Nanjing, China), human pulmonary cancer cell line (A549) was supplied by Dr Zhang Xu (Nanjing University of Chinese Medicine, China), human breast carcinoma cell line (MDA-MB-231), human colon carcinoma cell line (LoVo), human stomach poorly differentiated adenocarcinoma cell line (BGC-823), human gastric cancer cell line (SGC-7901, MKN-45), human ovarian carcinoma cells (A2780), were purchased from Cell Bank of Shanghai Institute of Cell Biology (Shanghai, China), maintained in RPMI 1640 culture medium plus 10% calf serum (Sijiqing Biological Engineer Material, Hangzhou, China) and100 U/mL penicillin, 100 μg/mL streptomycin (Gibco, USA), in a 37°C incubator supplied with 95% room air and 5% CO2. After confluency, the cells were trypsinize with 0.25% trypsin (AMRESCO, dissolved in PBS, pH 7.4), counted and placed down at a needed density for treatment.
Cell growth inhibitory ratio and cytotoxicity assay (MTT)
The cell survival rate and cytotoxicity were measured using 3-(4,5-dimethyl-2-thiazolyl)-2, 5-diphenyl tetrazolium bromide (MTT) assay. Briefly, exponentially growing SMMC- 7721, HeLa, A549, MDA-MB-231, LoVo, A2780, SGC- 7901, MKN-45, BGC-823 cells were seeded at a density of 6×104 cells/well in a 96-well tissue culture plate (Costar 3599, USA) with a total volume of 80 μL per well. After 40% confluency, cells were treated with brucine at various concentrations. MTT solution (5 mg/ml, dissolved in PBS, pH 7.4, 20 μl) was added to each well at 24h, 48h,72h of incubation and the cells were further cultured for 4h at 37°C, then the supernatant was discarded and 150 μL DMSO was added, the 96-well plate was vibrated on a micro-vibrator for an additional 10 min, the optical density of each well was measured at λ490 nm by Enzyme-immunoassay instrument (Bio-Rad, Model 680). The growth inhibitory ratio of each brucine was calculated based on the following formula: Growth inhibitory ratio (%) = (average A490 nm of control group average A490 nm of treated group)/ average A490 nm of control group × 100%. In this study, the drug concentration required to inhibit cell growth by 50% (IC50) was determined by interpolation from dose–response curves.
Apoptosis assessment by Hoechst33258 and AO/EB staining
The Hoechst 33258 staining was carried out as Hoechst Staining Kit described. Briefly, the A2780 cells in exponential growth were plated at a density of 5×104/ well in a 6-well chamber slide. After confluence, the cells were exposed to brucine. For Hoechst 33258 staining, the treated cells were fixed with fixative solution for 10 min, fixative solution was removed from the wells by aspiration and cells were washed twice with 0.9% NaCl and loaded with 500 μL Hoechst 33258 staining solution for 5min, then directly observed under a fluorescence microscope (Olympus, BX-60, Japan) in less than 20min. For AO/EB staining, the cells without fixation were loaded with 100 μl fresh-prepared AO/EB staining solution (100 μg/ml), then observed under a fluorescence microscope in less than 20 min.
DNA content analysis by flow cytometry
The DNA content of A2780 cells was determined by flow cytometry based on previously described method [6]. Briefly, A2780 cells were placed at a density of 1×106 cells/well in a 6-well plate. After treatment, cells were collected, washed twice with ice-cold PBS buffer (pH 7.4), fixed with 75% alcohol overnight, then stained with PI (1 mg/mL) in the presence of 10 mg/mL RNAse A for at least 30 min before analyzed with flow cytometry (Becton Dickinson, USA). The data were analyzed with Modift and CellQuest.
Determination of cell apoptosis by Annexin V and PI double staining
Apoptosis analyses were performed using the Annexin VFITC Apoptosis Detection Kit following the manufacturer’s instructions. Apoptosis and cell viability were measured by staining using annexin V-FITC and propidium iodide (PI), and the stained cells were analyzed immediately by fluorescence activated cell sorting (FACS) Calibur flow cytometry (Becton Dickinson, USA).
Statistical analysis
The statistical analysis involving two groups was performed by means of Student’s t-test, whereas analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test were used in order to compare more than two groups. All data were processed with SPSS software. Significance levels are reported as follows: *p < 0.05, **p < 0.01.
RESULTS
Growth inhibitory effects of brucine on nine cancer cell lines by MTT assay
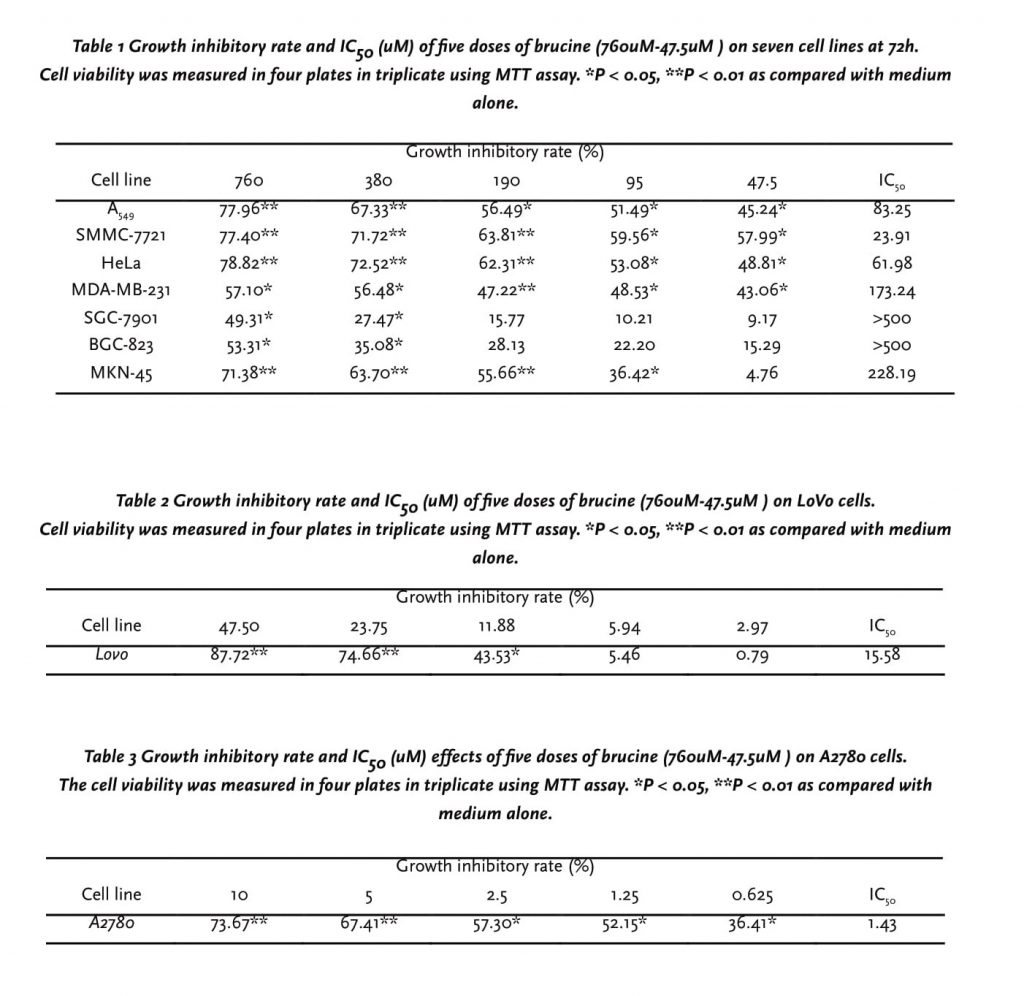
A dose-dependent inhibition in cell proliferation after 24h, 48h, 72h treatment by five doses of brucine was observed using the MTT assay. After 72h, the maximal effect on proliferation inhibition was observed with 760-47.5 μM brucine, and the growth inhibitory rate were all above 70.0% of LoVo cells and A2780 cells, so we decreased the dosage until found an obvious dose-dependent effect. As shown in table. 1, 2, 3, among 9 cell lines, A2780 cell line was the most sensitive cells to brucine, and the 50% inhibition concentration (IC50) of brucine for A2780 cells was 1.43μM, and the results indicated an obvious dose-dependent effect. BGC-823, SGC-7901 cells proved to be less sensitive to brucine, compared with other tumor cells.
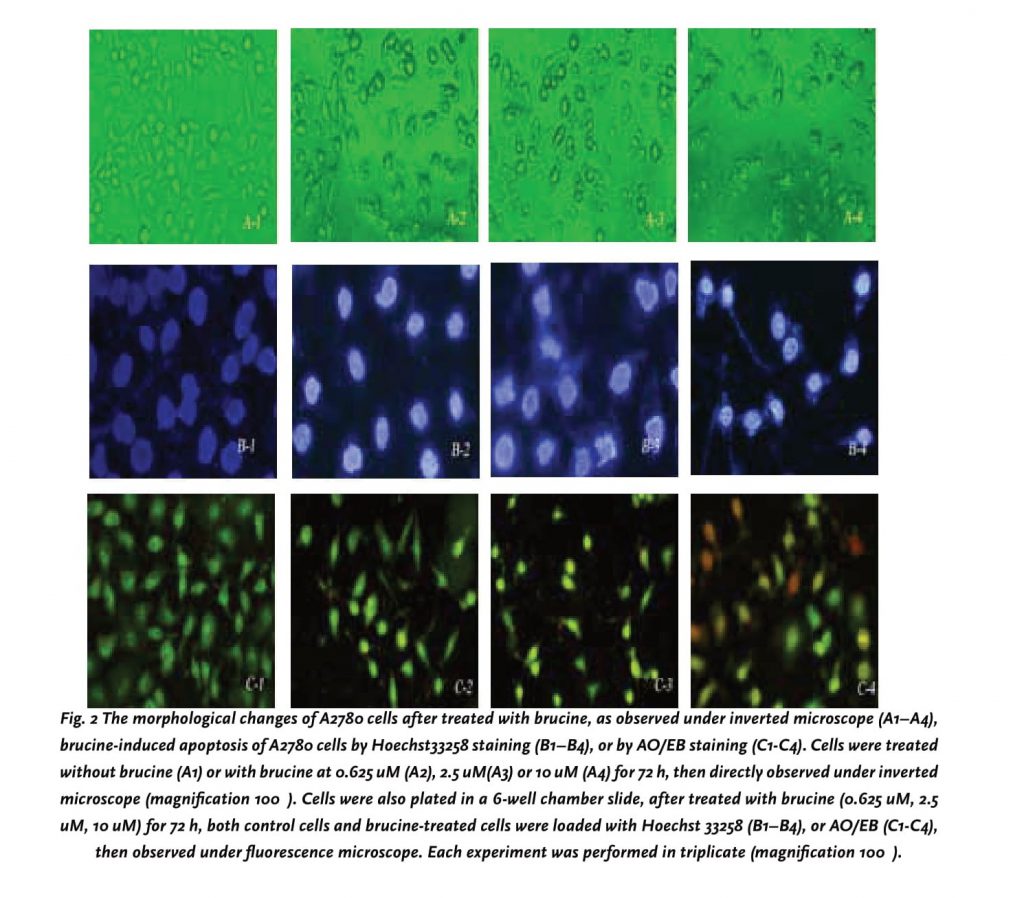
A2780 cells display apoptotic characteristics after treated with brucine
From above results, A2780 cells, among the nine tumor cell lines was shown to the most sensitive to brucine, thus, it was selected as a representative cell line to further investigate its cytotoxic mechanism. Firstly, cells after treated with brucine at different concentrations were directly observed under an inverted microscope at 72h. As shown in Figure 2A (1–4), A2780 cells after brucine treatment underwent significant morphological changes such as cells shrinkage, detachment from culture plate and formation of apoptotic bodies. To further address the death pattern, brucine treated A2780 cells were stained with Hoechst 33258 and AO/EB, respectively. All the cells and their nuclei were dyed blue when stained with Hoechst 33258 and brucine revealed marked nuclear condensation and fragmentation as well as the apoptotic bodies, all of which are typical characteristics of programmed cell death. Meanwhile, after staining with AO/EB (Figure 2C-3 and 2C-4) the apoptotic characteristic had been further confirmed in A780 cells after treated with brucine.
Flow cytometric analysis of brucine induced A2780 cells death
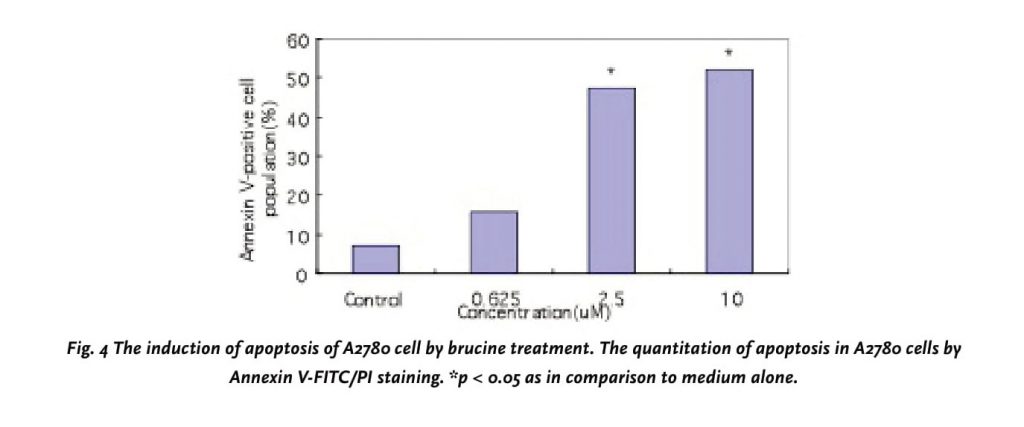
Using flow cytometric analysis of PI-stained fixed cells, we examined whether the cytotoxic effects of brucine were associated with cell cycle. We found that the apoptotic rates increased from 1.94% to 47.32% in a dose-dependent manner (0, 0.625, 2.5 and 10 μM) after 72h of processing (Table 4). The flow cytometry assay also showed an increase in the population of cells at the S phase, ranging from 28.65 to 38.47% in a dose-response manner, demonstrating that the cell cycle was being arrested at the S phase after the brucine treatment. On the other hand, an appearance of cells with DNA content less than G1 (sub-G1), characteristic of apoptotic cells, was observed at concentrations up to 0.625μM (Fig.3D2), and the percentage of sub-G1 increased in a dose-response manner (Fig.3D2-4). A quantitative evaluation of apoptosis was determined using an Annexin V-FITC dye to detect the translocation of phosphatidylserine from the inner (cytoplasmic) leaflet of the plasma membrane to the outer (cell surface). Compared with Control cell, 10 μM brucine induced 52.3% of apoptotic cells in A2780 at 72h (Fig. 4).
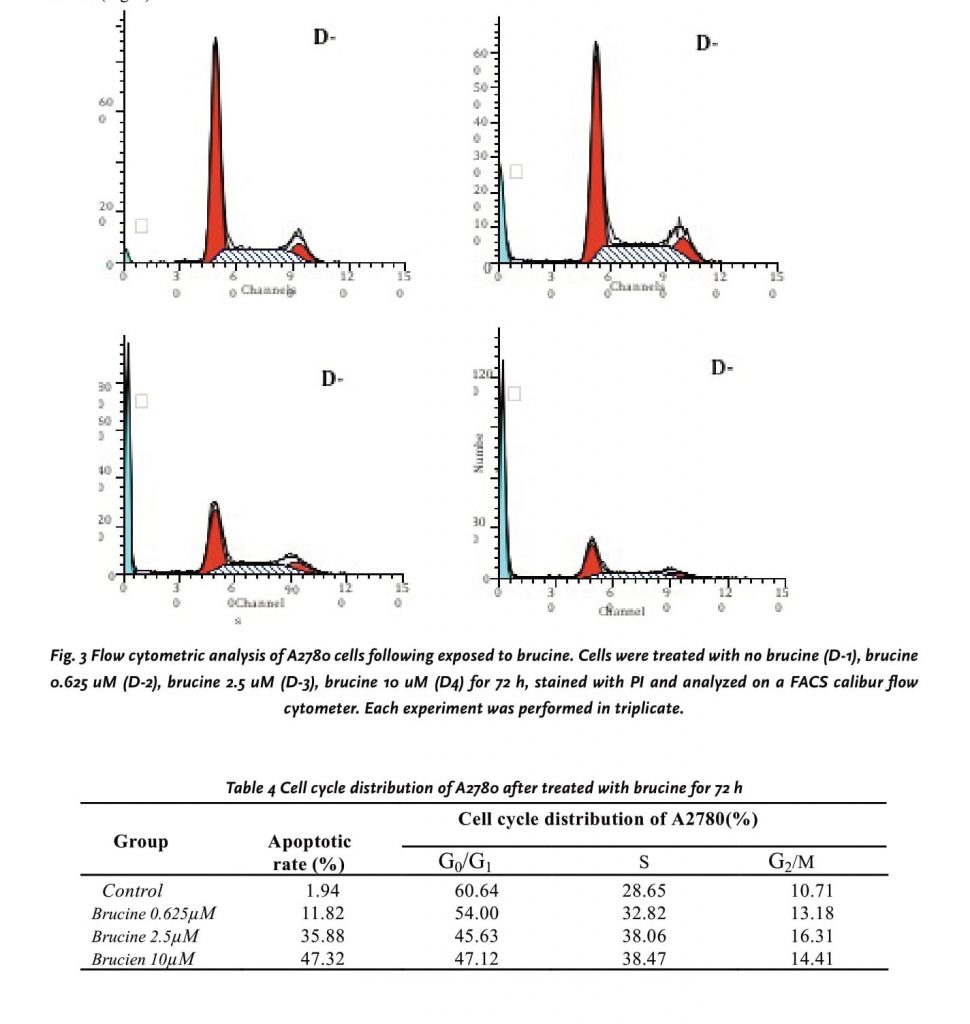
DISCUSSION AND CONCLUSIONS
In the present study, we investigated the cytotoxic effects of brucine from crude nux vomica on nine tumor cell lines. We found that among 9 cell lines, A2780 cell line was the most sensitive one to brucine, and the IC50 of brucine for A2780 cells after treatment for 72h was 1.43μM, that are approximately seventy-fold more sensitive than HepG2 cells, in detail, the IC50 effect of brucine on HepG2 cells proliferation after treatment for 72h was 0.1mM [7]. In addition, in this study, our data clearly demonstrate that brucine inhibits the proliferation of A2780 in a time and dosage-dependent manner. However, in comparison to other tumor cells, BGC-823, SGC-7901 cells proved to be less sensitive to the inhibitory effects of brucine, and the IC50 of brucine was more than 500 μM. This data suggested that not all tumor cells were sensitive to brucine. Therefore, we could choose more sensitive cells to examine the possible mechanisms. However, the effect of brucine, at least in this study, has been proven to be specific because brucine at 75 μM or higher didn’t cause significant apoptosis of human hepatic cells (L02) (data not shown).
Suppression of tumorigenesis often involves modulation of signal transduction pathways, leading to alterations in gene expression, arrest in cell cycle progression or apoptosis. Apoptosis represents a universal and exquisitely efficient suicide pathway, considered as an ideal way for elimination of damaged cells [8]. Recently, the apoptosis signaling systems have been shown to provide promising targets for the development of novel anticancer agents [9]. Several plant-derived bioactive agents are known to be chemo-preventive agents inducing apoptosis in a number of experimental models of carcinogenesis [10-12]. Thus, induction of apoptosis is considered as a possible mechanism of chemo-preventive agents. In the present study, cell cycle analysis employing flow cytometry demonstrated that brucine mainly caused an accumulation of A2780 cells in S phase and a minor accumulation in G2/M phase, which is not similar to the effect of brucine on HepG2 cells [7]. The results displayed the mechanism might be related to the influence of brucine in the cell cycle and the cell apoptosis induced by brucine. However, the progress in brucine-induced apoptosis in A2780 cells is still not fully clear and need to be further investigated, for example, if brucine could influence the protein level of Bcl-2, Bax and intracellular [Ca2+] in A2780 cells.
In our previous study, brucine was shown to relieve pain, reduce the release of inflammatory factors such as prostaglandin E2, thromboxane B2 and 6-keto-PGF1a in adjuvant-induced arthritis, the action mode of which was similar to the common NSAIDs [13]. Given the above facts, brucine could be a promising NSAID, which was effective in the treatment of ovarian cancer associated with pain or inflammation. Taken together, the final goal we are willing to achieve is to make brucine develop into an analgesic, anti-cancer new drug.
References
1. Xu, X.Y., Cai, B.C., Pan, Y., Wang, T.S. Pharmacokinetics of the alkaloids from the processed seeds of Strychnos nux-vomica in rats. Yao Xue Bao. 2003; 38(6): 458–461.
2. Yin, W., Jiang, G., Takeyasu, K., Zhou, X. Stimulation of Na, K-ATPase by low potassium is dependent on transferrin. J. Membr Biol. 2003; 193(3): 177-184
3. Cai, B. C., Hattori, M., and Namba, T. Processing of nux vomica. II. Changes in alkaloid composition of the seeds of Strychnos nux-vomica L. on traditional drug processing. Chem. Pharm. Bull. 1990; 38, 1295–1298.
4. Cai, B.C., Wang, T.S., Kurokawa, M., Shiraki, K., Hattori, M., Cytotoxicity of alkaloids from processed and unprocessed seeds of Strychnos nux-vomica. Acta Pharmacol. Sin. 1998; 19, 425–428.
5. Deng, X.K., Yin, F.Z., Lu, X.Y., Cai, B. C., Yin, W., The Apoptotic Effect of Brucine from the Seed of Strychnos nux-vomica on Human Hepatoma Cells is Mediated via Bcl-2 and Ca2+ Involved Mitochondrial Pathway. Toxicol. Sci. 2006; 91, 59–69.
6. Zauli, G., Sancilio, S., Cataldi, A., Sabatini, N., Bosco, D., Di Pietro, R., PI-3K/Akt and NF-kappaB/ Ikappaα pathways are activated in Jurkat T cells in response to TRAIL treatment. J. Cell. Physiol. 2005; 202, 900–911.
7. Deng, X.K., Yin, W., Li W. D., Yin, F.Z., Lu, X.Y, Zhang, X.C., Hua, Z.C., Cai, B.C. The anti-tumor effects of alkaloids from the seeds of Strychnos nux vomica on HepG2 cells and its possible mechanism. J Ethnopharmacol. 2006; 106, 179-186.
8. Singh, R.P., Dhanakakshmi, S. and Agarwal, R., Phytochemicals as cell cycle modulators: a less toxic approach in halting human cancer. Cell Cycle. 2002; 1, 156–161.
9. Hu, W. and Kavanagh, J.J. Anticancer therapy targeting the apoptotic pathway. Lancet Oncol. 2003. 4, 721–729. 10. Ahmad, N., Feyes, D.K., Nieminen, A.L., Agarwal, R. and Mukhtar, H., Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J. Natl. Cancer Inst. 1997; 89,1881–1886.
11. Lee, E. and Surh, Y.J. Induction of apoptosis in HL-60 cells by pungent vanilloids, [6]-gingerol and [6]-paradol. Cancer Lett, 1998; 134, 163-168.
12. Taraphdar, A.K., Roy, M. and Bhattacharya, R.K. Natural products as inducers of apoptosis: implication for cancer therapy and prevention. Curr. Sci., 2001; 80, 1387–1396.
13. Yin, W., Wang, T.S., Yin, F.Z., Cai, B.C., Analgesic and anti-inflammatory properties of brucine and brucine N-oxide extracted from seeds of Strychnox nux-vomica. J Ethnopharmacol. 2003; 88, 205–214.
ACKNOWLEDGEMENTS
This work was supported by National Nature Science Foundation of China (90209052, 30701111). The authors express thanks for the assistance from Professor Zhang Xu, Nanjing University of Traditional Chinese Medicine.
Correspondence to:
Baochang Cai, professor.
Nanjing University of Traditional Chinese Medicine, Nanjing 210029, China.
Telephone: +86-25-86798281.
Email: yinggao2006@126.com