Yoichi Matsui, Yasuo Kamiyama
First Department of Surgery, Kansai Medical University,
10-15 Fumizono, Moriguchi, Osaka 570-8507, Japan
ABSTRACT
OBJECTIVE
This retrospective study was conducted in terms of the prognosis of advanced breast cancer patients supplemented with Active Hexose Correlated Compound (AHCC).
METHODS
Out of 47 enrolled subjects, Stage I, II, III and IV were 5, 13, 10 and 19 cases respectively. Study period was six years from 1996 to 2002, and all subjects received AHCC administration in our department.
RESULTS
The prognosis of AHCC group was worse in comparison to that of the national counting in Stage I, II and III although a statistical work was impossible. AHCC supplementation improved the prognosis in Stage IV as compared to the national counting.
CONCLUSION
The results suggested that AHCC might contribute to improving the prognosis in Stage IV breast cancer patients although the improvement remains to be further elucidated in Stage I, II and III.
KEYWORDS
Breast cancer; Prognostic improvement effect; Survival rate; AHCC
Introduction
The incidence of breast cancer in Japan is relatively as little as about one-fifth compared with the Western countries. However, in recent years breast cancer tends to apparently increase depending on the change of food style and life environment and is ranked in third place after gastric and lung cancers in the cause of cancer death of Japanese women [1]. Breast cancer is early detectable by oneself, and a complete cure is also expectable if it is excised at an early stage. Breast cancer is a malignancy with relatively good prognosis as compared to other cancers. Unfortunately, even when a recurrence, it is effectively treated with radiotherapy [2, 3], various hormone therapies [4,5] and chemotherapy [6-8] whose varieties are also fast-evolving. Furthermore, a specific cancer immunotherapy has recently been developed, and the width of cancer therapy is spread. The immunotherapy [9,10] is first covered with a health insurance as cancer treatment. However, it is a fact that advanced recurrent breast cancer is mainly subjected to a supportive therapy and a treatment for QOL improvement, hardly expecting radical cure.
Our department focused on metastatic recurrent breast cancer patients undergoing several treatments or after treatments, and the hopefuls started the use of AHCC from May of 1996. This time, a retrospective study was conducted in terms of the prognosis of breast cancer patients supplemented with AHCC.
Methods
Subjects for the study
The study period was six years from May of 1996 to 2002, and the subjects were breast cancer patients who started AHCC administration in our department. The subjects at the age of 28 to 85 years received operative treatment of breast cancer during thirteen years from October, 1987 to September, 2000. Fifty-eight cases of breast cancer patients were treated with AHCC. Among these, 4 cases were inoperable patients due to progressive metastasis at the first visit, and because 7 cases underwent surgery at other hospitals, the stage and prognosis were unclear. The 11 cases were excluded, and residual 47 subjects were included. Out of 47 subjects, Stage I, II, III and IV were 5, 13, 10 and 19 cases, respectively.
This study was not a control study and had no comparable data. Therefore, the result was compared to the prognosis of breast cancer described in the investigation report of national breast cancer patient registration as a reference data.
Results
In this study, the commencing time of AHCC supplementation was different since AHCC was administered to the patients in accordance with their requests, not considering the period of operation, the stage of cancer and the check for recurrence. Some patients started to ingest AHCC just after the operation and others did ten years from post-operation. Besides, several patients began to take AHCC in the terminal care, resulting from recurrence and aggravation of general status. As a result, most patients commenced AHCC intake due to reappearance.
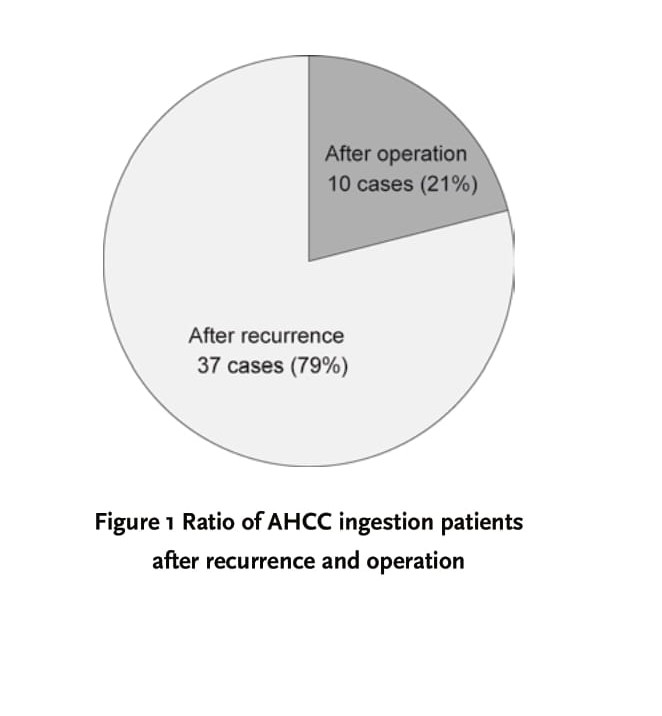
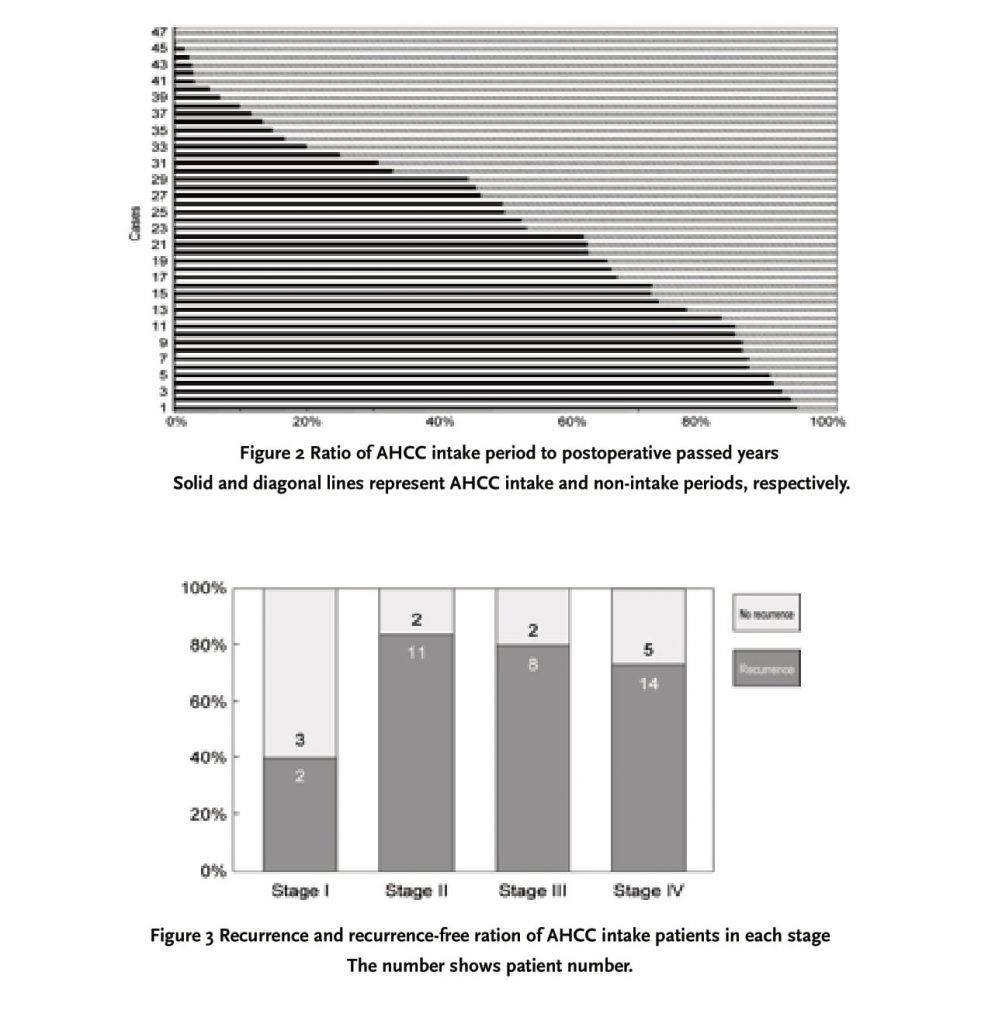
As shown in Figure 1, 10 cases (21%) out of 47 subjects began AHCC ingestion as part of a postoperative adjunctive therapy, while relapse made 37 cases (79%) to do it. There was a recurrence in 2 subjects (all Stage IV) of the 10 cases who started as the postoperative adjunctive therapy. Thus, 39 cases (83%) of the 47 patients available for analysis were recurrence patients. The period of AHCC supplementation following operation had great variation depending on the cases. Fig. 2 shows the ratio of AHCC intake period of each patient when postoperative passed years were estimated as 100%. Some patients immediately started taking AHCC after the operation, and others continued to do it for several months only. In Figure 2, it was demonstrated that AHCC ingestion period was approximately half of postoperative passed years as a whole. Eighty percent of beginning time of AHCC supplementation was corresponding to the relapsed time. Figure 3 indicates the rate of recurrence patients and recurrence-free ones in each stage. The ratio of relapsing patients was 40%, 85% 80% and 74% in Stage I, II, III and IV, respectively.
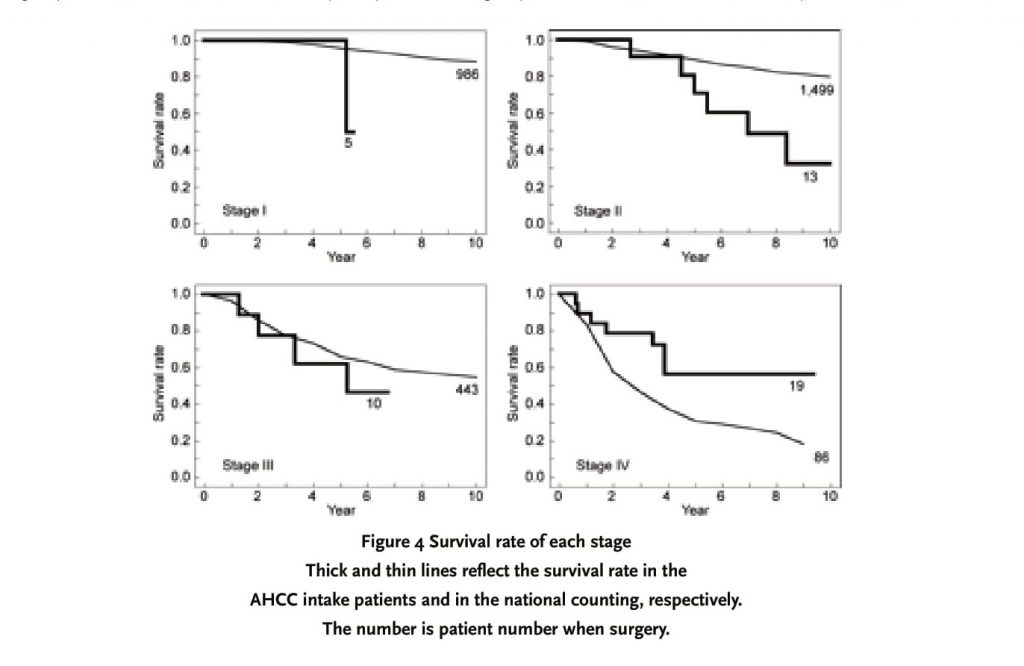
The survival rate of each stage was shown in Figure 4. The prognosis of breast cancer in the investigation report of national breast cancer patient registration was simultaneously presented as a reference data. In each stage, there is a significant difference of background factor between the population of the investigation report of national breast cancer patient registration and the AHCC supplementation group. Particularly, since the ratio of recurrence patients is very high in the group of AHCC intake, we cannot naively compare both groups. This is a reference data absolutely.
Discussion
As mentioned at the front, breast cancer shows very good prognosis in an early stage. The 10-year survival rate of post-operation in breast cancer is about 90%, 80%, 55% and 20% in Stage I, II, III and IV, respectively [2]. However, this retrospective study had many patients who visited our department in order to desire taking AHCC due to recurrence. Therefore, in the 47 cases available for analysis, the ratio of relapsing patients was high as an inevitable consequence (Fig. 1 and 3). The control group was not set up in the study and there is no general prognosis data of recurrence breast cancer patients alone, so that we used the post-operative prognosis of whole breast cancer based on the national counting as a reference data. Hence, the prognosis of AHCC supplementation group was worse than it. As shown in the survival curve of Fig. 4, the prognosis of AHCC group was worse compared to that of the national counting in Stage I, II and III although a statistical work was impossible. It is suggested that this might be attributed to the high ratio of recurrence patients of the AHCC group in the present study. In particular, Fig. 3 indicates that there was an apparently high tendency in the ratio of relapsing patients of Stage I, II and III. There is a difference in the observation period and no general data with respect to the ratio of recurrence patients in each stage, so we cannot compare exactly. If we consider that the 10-year survival rate of Stage I, II and III in the national counting is about 90%, 80% and 55%, respectively, it was found that the relapsing patient ratio in general Stage I, II and III was much lower than that of the AHCC group in this study. Therefore, it is thought that the prognosis of this study is worse compared with that of the national counting, because the ratio of recurrence patients was high in Phase I, II and III.
However, the notable point is that the AHCC group had a good result in Stage IV. In Stage IV, the prognosis from the national counting result is poor and the 10-year survival rate is around 20%. Consequently, the ratio of relapsing patients of Stage IV in the national counting must show a high level, considering that the ratio is similar to that of the AHCC group. Furthermore, since the patients of Stage IV had highly advanced cancer, the ratio of this stage patients who started taking AHCC immediately after operation but not after recurrence was higher than those of other stages. For these reasons, it seems that unlike other stages, Stage IV might maintain a balance in the background factor between the population of national counting and the patient group of this study. Thus, it is possible that AHCC supplementation improved the prognosis in Stage IV as compared to the national counting. However, in Stage IV as well as other stages, the case number was considerably different between the national counting (86 cases) and the AHCC group (19 cases), and the follow-up period was also different. Fig. 2 shows that the AHCC intake period was dependent on the cases. Although the data is not shown here, the period of AHCC supplementation in 19 patients of Stage IV had the width from 3 to 65 months depending on the cases, and the ratio of intake period to the postoperative passed years was also 7 to 100%. While 7 of 19 cases in Stage IV started AHCC supplementation immediately after operation, residual 12 cases did it after recurrence. Thus, as the variation in the commencing time and period of AHCC ingestion was extensive even in the same Stage IV, the present study only dose not make clear whether the difference of survival curve in Stage IV was attributed to AHCC supplementation alone.
The retrospective study in breast cancer patients with AHCC suggested that AHCC might contribute to improving the prognosis in Stage IV although the improvement remains to be elucidated in Stage I, II and III. Therefore, it is considered that a randomized controlled trial of AHCC in breast cancer patients is worthy enough to perform in the future.
References
[1] Tominaga S, Kuroishi T. Epidemiology and Prevention of Breast Cancer in the 21st Century. Breast Cancer 1999; 6: 283-288
[2] Bartelink H, Horiot JC, Poortmans P, Struikmans H, Van den Bogaert W, Barillot I, Fourquet A, Borger J, Jager J, Hoogenraad W, Collette L, Pierart M. Recurrence rates after treatment of breast cancer with standard radiotherapy with or without additional radiation. N Engl J Med 2001; 345: 1378-1387
[3] Pierce LJ. Treatment guidelines and techniques in delivery of postmastectomy radiotherapy in management of operable breast cancer. J Natl Cancer Inst Monogr 2001; 30: 117-124
[4] Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet 1998; 351: 1451-1467
[5] Goss PE, Strasser K. Aromatase inhibitors in the treatment and prevention of breast cancer. J Clin Oncol 2001; 19: 881-94
[6] Smorenburg CH, Bontenbal M, Verweij J. Capecitabine in breast cancer: current status. Clin Breast Cancer 2001; 1: 288-94
[7] Fogli S, Danesi R, Gennari A, Donati S, Conte PF, Del Tacca M. Gemcitabine, epirubicin and paclitaxel: pharmacokinetic and pharmacodynamic interactions in advanced breast cancer. Ann Oncol 2002; 13: 919-927
[8] Fumoleau P, Chauvin F, Namer M, Bugat R, Tubiana- Hulin M, Guastalla JP, Delozier T, Kerbrat P, Devaux Y, Bonneterre J, Filleul A, Clavel M. Intensification of adjuvant chemotherapy: 5-year results of a randomized trial comparing conventional doxorubicin and cyclophosphamide with high-dose mitoxantrone and cyclophosphamide with filgrastim in operable breast cancer with 10 or more involved axillary nodes. J Clin Oncol 2001; 19: 612-620
[9] Treish I, Schwartz R, Lindley C. Pharmacology, and therapeutic use of trastuzumab in breast cancer. Am J Health Syst Pharm 2000; 57: 2063-2079
[10] Sakamoto G, Mitsuyama S. New molecule-targeting therapy with herceptin (trastuzumab), an anti-HER2 (c-erB- 2) monoclonal antibody. Breast Cancer 2000; 7: 350-357.
Correspondence to:
Dr. Yoichi Matsui, MD
Department of Surgery, Kansai Medical University, 2-3-1 Shinmachi, Hirakara, Osaka 573-1191, Japan
Telephone: +81-72-804-0101
Fax: +81-72-804-2063
Email: matsui@Hirakata.kmu.ac.jp

