Brian Leung, ND, DC
Abstract
Vitamin C was a discovery that led to the prevention of scurvy. In modern science, its close resemblance in structure to glucose has enabled it to be recognized by cancer cells and made it successful in treating several cancers via intravenous routes in pharmacological doses. The mechanism and research behind how it converts to an ascorbyl radical and liberating hydrogen peroxide molecules that will create cytotoxicity for cancer cells will be explained. This exciting, revisited study on vitamin C via intravenous use prompts for more clinical studies for human use and which cancers this novel, yet age old, substance can be used for.
Keywords
Vitamin C; Ascorbic acid; Ascorbate; Intravenous ascorbic acid; Intravenous ascorbic; Ascorbic acid cancer; High dose ascorbic acid; Intravenous ascorbic acid cancer; Cancer; Hydrogen peroxide; Ascorbyl radical
Introduction
According to the Center for Disease Control in the U.S., we see a comparatively decrease in death rate from 1991 to 2006 in heart diseases (36%), cerebrovascular diseases (31%), influenza/pneumonia (49%), and cancers (16%). Cancers still the slowest in being affected by current social/scientific adjustments. In 2009, the American Cancer Society estimated cancer deaths are 292,540 for men and 269,800 for women, lung cancer being the most prevalent at 30% and 26%, respectively. This prompts for more radical, effective treatments to decrease the progression of cancers in our society. Natural alternatives to cancer therapies have been routinely sought out for and one question remains – is it effective for my cancer and have there been studies proving its validity?
Within natural medicine, we frequently hear of many miracle cures such as the Hoxsey Formula, Gerson Therapy, IV vitamin C, hyperthermia, and countless others. Many of these therapies are purely anecdotal and have not received proper funding to assess its truth to warrant a conclusion. Research on intravenous vitamin C has not been numerous enough to conduct a meta-analysis either. But within the last 20 years, studies have come out disproving the methods utilized in earlier studies conducted by the Mayo Clinic and Charles Moerte’s research on vitamin C as being non-effective on cancer when taken orally.1,2,3 In fact, ascorbate at pharmacologic concentrations, approximately 400 mg/dL, exhibits tumoricidal effects when administered intravenously.4,5,6 Given the mounting studies now available, we will discuss the potentiating effects of vitamin C as a parenteral nutrition.
Vitamin C, found most abundantly in glandular tissue, was first isolated in the 1930s by Albert Szent-Gyorgyi, who won the Nobel Prize for his discovery. Known as an anti-scorbutic factor, an antioxidant, an electron donor, an agent for pro-oxidant radicals and for the synthesis of neurotransmitters and other molecules, vitamin C has diametric functions within the body when certain conditions are present. Administered orally, vitamin C acts as an anti-oxidant and has a renal absorption threshold at 1.5 mg/dL in men and 1.3 mg/dL in women. Bowel absorption rate decreases as the intake of vitamin C increases. Maximum plasma ascorbate concentration using oral ascorbate can only reach 0.22 mM. On the other hand, vitamin C administered intravenously acts as a pro-oxidant when pharmacological concentrations are present. Plasma ascorbate concentration has been shown to range from 0.3 to 15 mM achievable only through IV, which demonstrates a 70-fold increase compared to oral absorption.7
Furthermore, the author of this review believes that the type of ascorbic acid given to the patient will also influence uptake into the extracellular fluid and subsequent diffusion into cancer cells. Studies have not proven or disproven this theory yet. Several forms are available, which may alter the kinetics of absorption: sodium ascorbate (pH 7.4), ascorbic acid (pH 4-5), L-ascorbic acid (i.e., from beets). The sodium ascorbate form is preferred in high dose applications in cancer because of its neutral pH and its ability to be less irritating to the veins when administered. A newer form of ascorbic acid, L-ascorbic acid, comes from beets and is available, but the difference in uptake of this form has not been validated. A study was also conducted using L-ascorbic acid-poly-D, L-(lactide-co-glycolide) nanoparticles containing violacein.8 The delivery system of nanoparticles demonstrated to be 2 times more efficient as an antitumoral compound compared to violacein by itself.
Mechanism of Action:
Intravenous Ascorbic Acid
The mechanism of action of intravenous ascorbic acid (IV AA) within the extracellular tissues makes it a pro-drug in its ability to kill cancer cells.9 Studies show taking ascorbic acid orally compared to intravenously differ substantially in its pharmacokinetics.
One study done by Muhlhofer demonstrated that 7.5g of intravenous vitamin C did not show an elevation of ascorbyl free radical in the blood.10 Qi Chen validated this fact, but points out that administration of IV AA forms an ascorbate radical (Asc•-) and H2O2 in the extracellular space, in vivo.11 The pharmacological concentration of ascorbyl radical needed in the extracellular fluid to create cytotoxicity in cancer cells is greater than 100mM, which can only be achieved via IV AA.
In the blood, a tightly controlled mechanism exists to limit the amount of ascorbate present. Chen has found that ascorbyl radical production in blood never exceeded >50 nM. Ascorbate is shuttled quickly to the extracellular space to generate an ascorbyl radical and subsequent H2O2 from electron donation. Any free radicals formed in the blood may quickly be disarmed by blood and plasma proteins.12 Thus, an acidotic state is never really formed.
Elaborating further, Chen states that, “the electron from ascorbate reduces a protein-centered metal,” where the reduced metal can donate an electron to oxygen and create superoxide radicals (O2•- or H2O2). The hydrogen peroxide formed can then diffuse across susceptible cancer cells creating cytotoxicity and cell death. The key to formation of hydrogen peroxide within the extracellular space is a metal-dependent enzyme, which interacts with ascorbate to form pro-oxidants in attacking cancer cells.13
An elevated level of copper that is needed in angiogenesis and the formation of cancer cells in various malignancies may be a clue to the underlying mechanism in oncogenesis. Investigating this association, a recent study has come out demonstrating the link of ascorbate to nuclear copper in its cytotoxic, redox mechanism in cancer cells.14 With iron- and copper -chelators present in the cells, the action of ascorbic acid was inhibited and did not cause DNA breakage through assays. This aptly demonstrates the crucial step, an iron- and copper- dependent enzyme, in forming pro-oxidants causing DNA breakage in cancer cells. Another study elucidated the same mechanism, a copper-/metal dependent enzyme that utilizes the chromatin bound copper, to produce free radicals that cause cytotoxicity for cancer cells.15 But what causes cancer cell death via apoptosis and pyknosis is dependent on the linear association of the reactant species, ascorbate in this case, in blood and extracellular fluid to H2O2 formed in the extracellular space.
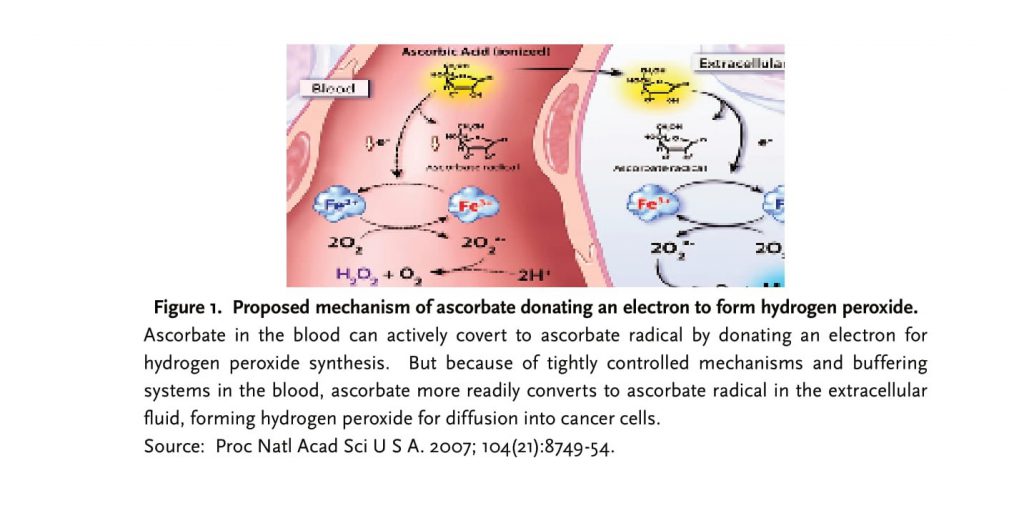
Intravenous Ascorbic Acid in Cancer
IV AA’s use as a parenteral agent for cancers is increasing.10, 16 The safety of IV AA use has been studied in phase I clinical trials demonstrating minimal adverse events and toxicity at all dose levels.17 In cell culture studies, vitamin C has proven to be useful in suppressing cancer growth.18 Phase II clinical trials on intravenous vitamin C are underway given its substantiated claims and safety.19, 20, 21, 22 Various other studies have found its efficacy in patients with many types of malignancies including but not limited to acute myeloid leukemia/myelodyspastic syndromes23, ovarian10, pancreatic10, 24, glioblastoma10, renal cell carcinoma25 , adenocarcinoma26 small cell carcinoma of the lung27, bladder cancer31, mesothelioma28, and other cancers. Additional case reports done by Hugh D. Riordan and others show the effectiveness of intravenous vitamin C in cancer but lack completeness in their study.
A phase I clinical trial shows that IV AA has no objective anticancer response on advanced malignancy.18 In another phase II clinical trial, arsenic trioxide in combination with intravenous ascorbic acid and temozolomide had no response in metastatic melanoma.29 Though, this trial only infused 1 g of ascorbic acid after administration of arsenic trioxide, which would not generate the pharmacologic concentration needed for cytotoxicity in cancer cells as researched by Chen.
Safety of Intravenous Ascorbic Acid
Intravenous vitamin C is the most widely used in complementary oncology, but its increasing use may necessitate its safety precaution.7, 30, 31 Some complementary practitioners have claimed administering dosages higher than 100 g over several hours, granted its safe history. Typically, ascorbate stays within the body for a few hours before being excreted from the kidneys via formation of oxalic acid. Though, IV AA may have adverse effects such as dehydration, hypoglycaemia, nausea, vomiting, and shakiness. Some of these symptoms may be correlated to poor kidney function and low serum calcium. Therefore, a baseline workup should be established before initiating treatment. Ascorbic acid is also lower in pH than other forms and may cause more irritation to the veins in high concentration. Contraindications to using IV AA include: congestive heart failure, ascites, edema, renal insufficiency, chronic haemodialysis patients, iron overload, and possibly oxalate storm formers. Special precaution should be taken when using the sodium ascorbate form in patients with CHF or renal disease. In addition, intravenous vitamin C use in glucose-6-phoshate dehydrogenase (G6PD) deficiency may cause hemolysis.
Discussion
With the amount of research already done on vitamin C it is abundantly clear that IV AA has some use in treating cancers. Quite a few studies that were referred to and conducted but failed clinical trials did not reach pharmacological concentrations of IV AA that was investigated to be tumoricidal. Its ability in pharmacologic concentrations, approximately 100 mM, will create an ascorbyl radical and subsequent electron donation for free radical production within the extracellular matrix. The specificity in selection of hydrogen peroxide, via diffusion, within cancer cells creates cell death, while sparing normal cells.
A few details still need to be investigated and elucidated about the mechanism of action of ascorbic acid and its action on tumors. For example, how does ascorbic acid selectively target only tumor cells? Through what transport mechanism does it go through to cause cytotoxicity? What factors mediate H2O2 production? And what specific cancers is IV AA used for? Understanding these questions will better allow physicians to readily prescribe IV AA as a concurrent and foremost treatment when dealing with cancer and prolonging survival times. IV AA is still not FDA approved and is currently undergoing clinical trials. To date, research already done on IV AA for cancer shows a promising novel treatment in cancer. My theory is that cancers that utilize glucose uptake the most will be the ones that will be most affected by IV AA.
References
1. Moertel CG, Fleming TR, Creagan ET, et al. High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy. A randomized double-blind comparison. N Engl J Med. 1985;312(3):137-41.
2. Creagan ET, Moertel CG, O’Fallon JR, et al. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N Engl J Med. 1979; 301(13):687-90.
3. Padayatty SJ, Levine M. Reevaluation of ascorbate in cancer treatment: emerging evidence, open minds, and serendipity. J Am Coll Nutr 2000; 19:423-5.
4. Duconge J, Miranda-Massari JR, Gonzalez MJet, al. Pharmacokinetics of vitamin C: insights into the oral and intravenous administration of ascorbate. P R Health Sci J. 2008; 27(1):7-19. Review.
5. Riordan HD, Riordan NH, Meng X, et al. Improved microplate fluorometer counting of viable tumor and normal cells. Anticancer Res. 1994; 14(3A):927-31.
6. Riordan NH, Riordan HD, Meng X, Li Y, Jackson JA. Intravenous ascorbate as a tumor cytotoxic chemotherapeutic agent. Med Hypotheses. 1995; 44(3):207-13.
7. Chen Q, Espey MG, Krishna MC, et al. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. Proc. Natl. Acad. Sci. U.S.A. 2005; 102 (38): 13604–9.
8. Martins D, Frungillo L, Anazzetti MC, et al. Antitumoral activity of L-ascorbic acid-poly- D, L-(lactide-co-glycolide) nanoparticles containing violacein. Int J Nanomedicine. 2010; 5:77-85.
9. Chen Q, Espey MG, Sun AY, et al. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci U S A. 2008; 105(32):11105-9.
10. Muhlhofer A, Mrosek S, Schlegel B, et al. High-dose intravenous vitamin C is not associated with an increase of pro-oxidative biomarkers. Eur J Clin Nutr 2004; 58:1151–1158.
11. Chen Q, Espey MG, Sun AY, et al. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci U S A. 2007; 104(21):8749-54.
12. Johnson RM, Goyette G, Jr., Ravindranath Y, et al. Free Radical Biol Med. 2005; 39:1407–1417.
13. Frei B, Lawson S. Vitamin C and cancer revisited. Proc Natl Acad Sci U S A. 2008; 105(32):11037-8.
14. Ullah MF, Khan HY, Zubair H, et al. The antioxidant ascorbic acid mobilizes nuclear copper leading to a prooxidant breakage of cellular DNA: implications for chemotherapeutic action against cancer. Cancer Chemother Pharmacol. 2010 Mar 6.
15. Hadi SM, Ullah MF, Azmi AS, et al. Resveratrol Mobilizes Endogenous Copper in Human Peripheral Lymphocytes Leading to Oxidative DNA Breakage: A Putative Mechanism for Chemoprevention of Cancer. Pharm Res. 2010; 27(6): 979-88.
16. Verrax J, Calderon PB. Pharmacologic concentrations of ascorbate are achieved by parenteral administration and exhibit antitumoral effects. Free Radic Biol Med. 2009; 47(1):32-40.
17. Hoffer LJ, Levine M, Assouline S, et al. Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann Oncol. 2008; 19(11):1969-74.
18. Park CH, Kimler BF, Bodensteiner D, et al. In vitro growth modulation by L-ascorbic acid of colony-forming cells from bone marrow of patients with myelodysplastic syndromes. Cancer Res. 1992; 52(16):4458-66.
19. http://clinicaltrials.gov/ct2/show/NCT00626444
20. http://clinicaltrials.gov/ct2/show/NCT01050621
21. http://clinicaltrials.gov/ct2/show/NCT00006021
22. http://clinicaltrials.gov/ct2/show/NCT00284427?te rm=Intravenous+Vitamin+C&rank=4
23. Park CH, Kimler BF, Yi SY, et al. Depletion of L-ascorbic acid alternating with its supplementation in the treatment of patients with acute myeloid leukemia or myelodysplastic syndromes. Eur J Haematol. 2009; 83(2):108-18.
24. Jackson JA, Riordan HD, Hunninghake RE, et al. Highdose intravenous vitamin C and long-time survival of a patient with cancer of the head of the pancreas. J Orthomol Med 1995; 10:87-8.
25. Riordan HD, Jackson JA, Riordan NH, et al. Highdose intravenous vitamin C in the treatment of a patient with renal cell carcinoma of the kidney. J Orthomol Med 1998; 13:72-3.
26. Riordan HD, Jackson JA, Schultz M. Case study: highdose intravenous vitamin C in the treatment of a patient with adenocarcinoma of the kidney. J Orthomol Med 1990; 5:5-7.
27. Padayatty SJ, Riordan HD, Hewitt SM, et al. Intravenously administered vitamin C as cancer therapy: three cases. CMAJ. 2006; 174(7):937-42.
28. Takemura Y, Satoh M, Satoh K, et al. High dose of ascorbic acid induces cell death in mesothelioma cells. Biochem Biophys Res Commun. 2010; 394(2): 249-53.
29. Bael TE, Peterson BL, Gollob JA. Phase II trial of arsenic trioxide and ascorbic acid with temozolomide in patients with metastatic melanoma with or without central nervous system metastases. Melanoma Res. 2008; 18(2):147-51.
30. Grober U. Vitamin C in complementary oncology– update 2009. Med Monatsschr Pharm. 2009; 32(7):263-7. Review. German.
31. Stephenson CM, Levin RD, Lis CG. Phase 1 trial of high-dose intravenous vitamin C treatment for patients with cancer. J Am Osteopath Assoc. 2007; 107(6):212-3.
Figure 1: Chen Q, Espey MG, Sun AY, et al. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci U S A. 2007; 104(21):8749-54.
Correspondence to:
Dr. Brian Leung, ND, DC
Telephone: (604) 303-9952
Fax: (604) 303-9926
Email: brianhleung@gmail.com

